Impacts of temperature on recombination rate and meiotic success in thermotolerant and cold-tolerant yeast species
- PMID: 40715475
- PMCID: PMC12316985
- DOI: 10.1038/s41437-025-00778-6
Impacts of temperature on recombination rate and meiotic success in thermotolerant and cold-tolerant yeast species
Abstract
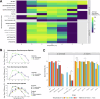
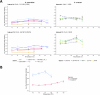
Meiosis is required for the formation of gametes in all sexually reproducing species and the process is well conserved across the tree of life. However, meiosis is sensitive to a variety of external factors, which can impact chromosome pairing, recombination, and fertility. For example, the optimal temperature for successful meiosis varies between species of plants and animals. This suggests that meiosis is temperature sensitive, and that natural selection may act on variation in meiotic success as organisms adapt to different environmental conditions. To understand how temperature alters the successful completion of meiosis, we utilized two species of the budding yeast Saccharomyces with different temperature preferences: thermotolerant Saccharomyces cerevisiae and cold-tolerant Saccharomyces uvarum. We surveyed three metrics of meiosis: sporulation efficiency, spore viability, and recombination rate in multiple strains of each species. As per our predictions, the proportion of cells that complete meiosis and form spores is temperature sensitive, with thermotolerant S. cerevisiae having a higher temperature threshold for completion of meiosis than cold-tolerant S. uvarum. We confirmed previous observations that S. cerevisiae recombination rate varies between strains and across genomic regions, and add new results that S. uvarum has comparably high recombination rates. We find significant recombination rate plasticity due to temperature in S. cerevisiae and S. uvarum, in agreement with studies in animals and plants. Overall, these results suggest that meiotic thermal sensitivity is associated with organismal thermal tolerance and may even result in temporal reproductive isolation as populations diverge in thermal profiles.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Research ethics statement: Not needed.
Figures
Update of
-
Temperature affects recombination rate plasticity and meiotic success between thermotolerant and cold tolerant yeast species.bioRxiv [Preprint]. 2024 Aug 29:2024.08.28.610152. doi: 10.1101/2024.08.28.610152. bioRxiv. 2024. Update in: Heredity (Edinb). 2025 Aug;134(8):473-484. doi: 10.1038/s41437-025-00778-6. PMID: 39257736 Free PMC article. Updated. Preprint.
Similar articles
-
Temperature affects recombination rate plasticity and meiotic success between thermotolerant and cold tolerant yeast species.bioRxiv [Preprint]. 2024 Aug 29:2024.08.28.610152. doi: 10.1101/2024.08.28.610152. bioRxiv. 2024. Update in: Heredity (Edinb). 2025 Aug;134(8):473-484. doi: 10.1038/s41437-025-00778-6. PMID: 39257736 Free PMC article. Updated. Preprint.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
CAYSS: Package for Automatic Cytometry Analysis of Yeast Spore Segregation.Yeast. 2024 Nov;41(11-12):681-690. doi: 10.1002/yea.3988. Epub 2025 Jan 22. Yeast. 2024. PMID: 39844477 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Aggarwal DD, Rybnikov S, Cohen I, Frenkel Z, Rashkovetsky E, Michalak P, Korol AB (2019) Desiccation-induced changes in recombination rate and crossover interference in Drosophila melanogaster: evidence for fitness-dependent plasticity. Genetica 147(3):291–302. 10.1007/s10709-019-00070-6. - PubMed
-
- Almeida P, Gonçalves C, Teixeira S, Libkind D, Bontrager M, Masneuf-Pomarède I, Albertin W, Durrens P, Sherman DJ, Marullo P, Todd Hittinger C, Gonçalves P, Sampaio JP (2014) A Gondwanan imprint on global diversity and domestication of wine and cider yeast Saccharomyces uvarum. Nat Commun 5: 4044. 10.1038/ncomms5044. - PMC - PubMed
-
- Bayliss MW, Riley R (1972) An analysis of temperature-dependent asynapsis in Triticum aestivum. Genet Res 20(2):193–200. 10.1017/S0016672300013707.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

