Establishment and characterization of a novel patient-derived TP63-rearranged anaplastic large-cell lymphoma model PTCL-S1
- PMID: 40715645
- PMCID: PMC12296843
- DOI: 10.1007/s13577-025-01264-1
Establishment and characterization of a novel patient-derived TP63-rearranged anaplastic large-cell lymphoma model PTCL-S1
Abstract
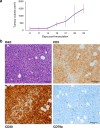
Anaplastic large-cell lymphoma (ALCL) accounts for 15% of all peripheral T-cell lymphomas globally and can be further divided into subcategories, of which patients with ALK-negative ALCL have dismal prognosis and overall survival. We established a patient-derived xenograft (PDX) and in vitro model (designated PTCL-S1) of TP63-rearranged ALK-negative ALCL from the primary tumour site of a 55-year old Chinese woman. Whole genome sequencing of the patient's tumour identified various mutations including AKT1 and NOTCH1, as well as the TP63-TBL1XR1 gene fusion. RNA sequencing followed by Sanger sequencing confirmed the gene rearrangement in original tumour, PDX and PTCL-S1 cell line. Immunohistochemistry profiling of the PDX model and cell-line were consistent with the patient's primary tumour sample (CD3 + /CD30 + /CD79a-). Cytotoxic agents (doxorubicin, etoposide and gemcitabine) commonly used in ALCL treatment exhibited potent anti-proliferative activity in the cell-line. In conclusion, the established PTCL-S1 cell line can be a useful tool for further investigation of the understanding of TP63-rearranged ALK-negative ALCL.
Keywords: Anaplastic large cell lymphoma (ALCL); Patient-derived xenograft; TP63 rearrangement; TP63-TBL1XR1 fusion; Tumour models.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: The authors declare no competing interests. Ethical approval: Ethics approval from the SingHealth Centralized Institution Review Board was obtained for tissue collection and consent protocols (CIRB 2018/3084). Xenograft studies were conducted in compliance with animal protocols approved by the SingHealth Institutional Animal Care and Use Committee (IACUC) (2018/SHS/1371). Informed consent: Written informed consent from the patient for use of clinical data and biospecimens was obtained in accordance with the Declaration of Helsinki.
Figures




Similar articles
-
Anaplastic large cell lymphoma: unusual clinical and pathologic findings: A report of the 2023 SH-EA4HP Lymphoma Workshop.Am J Clin Pathol. 2025 Jul 11;164(1):110-125. doi: 10.1093/ajcp/aqaf029. Am J Clin Pathol. 2025. PMID: 40646678
-
Genomics of T-Cell Lymphomas With CD30/CD15 Co-Expression: Comparison With Anaplastic Large-Cell Lymphoma, ALK-Negative.Am J Surg Pathol. 2025 Jul 3. doi: 10.1097/PAS.0000000000002447. Online ahead of print. Am J Surg Pathol. 2025. PMID: 40605272
-
Aberrant expression and genetic alteration of c-MYC in anaplastic large cell lymphoma.J Cancer Res Clin Oncol. 2022 Jan;148(1):267-278. doi: 10.1007/s00432-021-03691-7. Epub 2021 Jun 16. J Cancer Res Clin Oncol. 2022. PMID: 34131801 Free PMC article.
-
Anaplastic large cell lymphoma in children and adolescents.Br J Haematol. 2025 Aug;207(2):336-349. doi: 10.1111/bjh.20154. Epub 2025 May 12. Br J Haematol. 2025. PMID: 40351161 Free PMC article. Review.
-
Targeted therapy for advanced anaplastic lymphoma kinase (<I>ALK</I>)-rearranged non-small cell lung cancer.Cochrane Database Syst Rev. 2022 Jan 7;1(1):CD013453. doi: 10.1002/14651858.CD013453.pub2. Cochrane Database Syst Rev. 2022. PMID: 34994987 Free PMC article.
References
-
- O’Connor OA, Ma H, Chan JYS, Kim SJ, Yoon SE, Kim WS. Peripheral T-cell lymphoma: from biology to practice to the future. Cancer Treat Rev. 2024;129:102793. 10.1016/j.ctrv.2024.102793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

