The source of dietary fat influences anti-tumour immunity in obese mice
- PMID: 40715760
- PMCID: PMC12373505
- DOI: 10.1038/s42255-025-01330-w
The source of dietary fat influences anti-tumour immunity in obese mice
Abstract
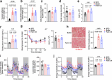
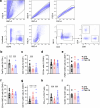
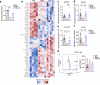
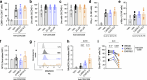
Obesity increases the risk of many cancers and impairs the anti-tumour immune response. However, little is known about whether the source or composition of dietary fat affects tumour growth or anti-tumour immunity in obesity. Here, we show that high-fat diets (HFDs) derived from lard, beef tallow or butter accelerate tumour growth in a syngeneic model of melanoma, but HFDs based on coconut oil, palm oil or olive oil do not, despite equivalent obesity. Using butter-based and palm oil-based HFDs as examples, we find that these dietary fat sources differentially regulate natural killer and CD8 T cell infiltration and function within the tumour microenvironment, governed by distinct effects on the plasma metabolome and intracellular metabolism. We identify diet-related lipid intermediates, namely long-chain acylcarnitine species, as immunosuppressive metabolites enriched in mice fed butter compared to palm oil HFD. Together, these results highlight the significance of diet in maintaining a healthy immune system and suggest that modifying dietary fat may improve cancer outcomes in obesity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: L.L. is a member of the scientific advisory board for MiNK Therapeutics, a consultant for Bayer and a member of the Scientific Advisory Board of Faeth Therapeutics and Deciduous Therapeutics. The interests of L.L. were reviewed and managed by the Brigham and Women’s Hospital in accordance with their conflict-of-interest policies. M.H. is a member of the Scientific Advisory Board of the MD Anderson Allison Institute. The other authors declare no competing interests.
Figures







References
-
- World obesity day 2022—accelerating action to stop obesityhttps://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelera... (2022).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

