Identifying and assessing the capacity and experience of trial sites in low- and middle-income countries for high-quality randomised drug trials in maternal and perinatal health
- PMID: 40716797
- PMCID: PMC12306368
- DOI: 10.1136/bmjgh-2024-018063
Identifying and assessing the capacity and experience of trial sites in low- and middle-income countries for high-quality randomised drug trials in maternal and perinatal health
Abstract
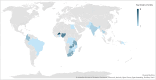
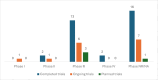
Existing international consortia for drug trials in maternal and perinatal health have focused largely on pragmatic trials using off-label medicines. This study aimed to identify and assess the capacity and experience of sites in low- and middle-income countries (LMICs) for conducting trials for regulatory approval of medicines for pregnancy-related conditions. We systematically reviewed site assessment checklists across any disease area to develop a maternal trial site assessment checklist. The checklist was pretested, revised and used to collect data from trial sites in LMICs. Sites were systematically identified from a scoping review of maternal trials conducted in LMICs, known networks and snowball searching. Data reported by sites were verified against publicly accessible sources (clinical trial registries and published articles). We contacted 106 sites in 30 countries, of which 49 (46.2%) sites in 21 countries completed the checklist. Sites were from five regions-Sub-Saharan Africa (37), South Asia (6), Latin America and the Caribbean (4), Middle East and North Africa (1) and East Asia and the Pacific (1). More than 70% of responding sites had the requisite physical infrastructure, clinical and research staff, ethics, participant recruitment and data management services to conduct randomised trials. Respondents collectively identified 52 completed, ongoing or planned maternal trials across their sites. Of these 52 trials, 16 (30.8%) were Good Clinical Practice-compliant, 22 (42.3%) were phase III and one was a regulatory trial. 14 trials were conducted by a collaborative group established mostly for a specific trial or a small group of related trials. Only two of these groups were pre-established trial networks. While there is some capacity to conduct high-quality maternal drug trials in LMICs, effective research collaborations are needed to further strengthen and expand this capacity. Establishing a sustainable LMIC-based trial network will accelerate the development and testing of novel drugs to improve maternal and newborn health outcomes in these regions.
Keywords: Epidemiology; Global Health; Health services research; Obstetrics; Public Health.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
The Impact of Infrastructure on Low-Income Consumers' Nutritious Diet, Women's Economic Empowerment, and Gender Equality in Low- and Middle-Income Countries: An Evidence and Gap Map.Campbell Syst Rev. 2025 Jul 18;21(3):e70050. doi: 10.1002/cl2.70050. eCollection 2025 Sep. Campbell Syst Rev. 2025. PMID: 40688267 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Use of biochemical tests of placental function for improving pregnancy outcome.Cochrane Database Syst Rev. 2015 Nov 25;2015(11):CD011202. doi: 10.1002/14651858.CD011202.pub2. Cochrane Database Syst Rev. 2015. PMID: 26602956 Free PMC article.
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- World Health Organization . Trends in maternal mortality 2000 to 2020: estimates by WHO, UNICEF, UNFPA, World Bank Group and UNDESA/population division, licence: CC BY-NC-SA 3.0 IGO. Geneva: 2023.
-
- Birmingham, UK: University of Birmingham, Birmingham Health Partners; 2022. Healthy mum, healthy baby, healthy future: the case for UK leadership in the development of safe, effective and accessible medicines for use in pregnancy.
-
- AIM . Geneva, Switzerland: 2020. Medicines for pregnancy-specific conditions: research, development and market analysis.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical