Multi-omics analysis of lactate metabolism gene regulation in Clonorchis sinensis-associated hepatocellular carcinoma
- PMID: 40717080
- PMCID: PMC12302829
- DOI: 10.1186/s13071-025-06947-0
Multi-omics analysis of lactate metabolism gene regulation in Clonorchis sinensis-associated hepatocellular carcinoma
Abstract
Background: Although recent research has highlighted lactylation, a post-translational modification driven by elevated lactate levels, as a critical regulator of key cellular pathways in hepatocellular carcinoma (HCC), its contribution to the poor prognosis of Clonorchis sinensis (Cs)-infected HCC remains poorly understood.
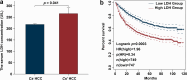
Methods: We first identified the significant upregulation of the lactate metabolism enzyme LDH in Cs-infected HCC patients through clinical retrospective analysis. We then conducted a multi-omics analysis (RNA-Seq, ATAC-Seq, WGBS-Seq, oxWGBS-Seq, and ChIP-Seq) to examine the differences in 392 lactate metabolism-related genes (LMRGs) between Cs-infected and Cs-noninfected HCC tumors. Six key differentially expressed LMRGs were further validated using RT-qPCR assays to confirm their expression and potential role in HCC progression.
Results: The differential expression levels of 8 LMRGs, along with 71 accessible regions and 42 CpG sites in the promoters of LMRGs, were identified. Notably, we also demonstrated that histone modifications, including H3K9ac, H3K79me2, H3K4me2, H3K4me3, H3K27ac, and H3K4me1, were associated with chromatin accessibility in the promoters of LMRGs. Finally, the TCGA-LIHC cohort confirmed that the differential expression of LMRGs between Cs-infected and Cs-noninfected HCC tumors significantly affects the survival outcomes of HCC.
Conclusions: Our findings revealed that lactylation plays an important role in reshaping the characteristics of HCC during Cs infection, expanding our understanding of the unique features of Cs-infected HCC.
Keywords: Clonorchis sinensis; Hepatocellular carcinoma; Lactate metabolism; Multi-omics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committees of the Affiliated Cancer Hospital of Guangxi Medical University (KY2025016) and conducted in accordance with the Declaration of Helsinki. Upon admission, all patients provided written consent for the analysis and publication of their anonymized medical data for research purposes. Competing interests: The authors declare no competing interests. Consent for publication: Not applicable.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

