Hydrogels as advanced drug delivery platforms for cancer immunotherapy: promising innovations and future outlook
- PMID: 40717098
- PMCID: PMC12302882
- DOI: 10.1186/s12951-025-03613-6
Hydrogels as advanced drug delivery platforms for cancer immunotherapy: promising innovations and future outlook
Abstract
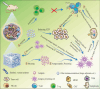
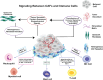
Tumor immunotherapy has appeared as a groundbreaking method in cancer therapy, which destroys cancer cells through identification and attack by stimulating the body's immune system. Despite its rapid development, there are serious challenges to overcome. Efficient delivery of the immunotherapeutic cargos to the tumor microenvironment (TME), activation, and systemic adverse reactions have hindered their therapeutic application. Due to their biocompatibility, self-healing ability, and stable localized drug delivery to the tumor niche, hydrogels are regarded as potent delivery platforms. Tailor-made 3D hydrogels from various polymers have shown high drug-loading capacity, which could deliver different immunomodulators to activate effector T cells and enhance immunotherapy efficiency. Injectable hydrogels have also gained significant attention as carriers for tumor vaccines and cell delivery due to their minimal invasiveness encapsulation of various immunotherapeutics, protecting them from degradation and triggering a local immune response. This article reviews recent advances in using hydrogels as immunotherapeutic and cell delivery platforms in cancer immunotherapy, highlighting their ability to overcome the limitations of current delivery systems. In addition, their structure and functional modifications in the development of stimuli-responsive hydrogels, injectable and multifunctional hydrogels are further discussed. Prospects and obstacles in the development of hydrogel-based cancer immunotherapy are also examined.
Keywords: Cancer immunotherapy; Hydrogels; Injectable; Stimuli-responsive; Tumor vaccines.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

