Toxic epidermal necrolysis following lamotrigine replacement therapy in a woman planning pregnancy: a case report and literature review
- PMID: 40717110
- PMCID: PMC12302893
- DOI: 10.1186/s12905-025-03796-y
Toxic epidermal necrolysis following lamotrigine replacement therapy in a woman planning pregnancy: a case report and literature review
Abstract
Background: Lamotrigine is a preferred antiepileptic drug for women with epilepsy planning or undergoing pregnancy, owing to its relatively low teratogenic risk. However, serious adverse reactions, including life-threatening conditions such as Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), can occur.
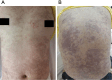
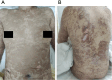
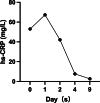
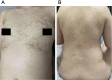
Case presentation: We report the case of a 33-year-old woman of childbearing age with epilepsy who developed TEN following the initiation of lamotrigine as part of a preconception antiepileptic regimen. The patient presented with high fever, a rapidly spreading erythematous rash, bullae formation, and extensive epidermal detachment. Prompt and comprehensive multidisciplinary treatment led to significant clinical improvement and eventual full recovery. The therapeutic approach included intravenous dexamethasone sodium phosphate, fluid resuscitation, intravenous immunoglobulin (IVIG), oral antihistamines, traditional Chinese medicine (TCM), and meticulous care of the skin and mucosal surfaces.
Conclusion: While antiepileptic drugs (AEDs) such as lamotrigine are crucial for managing epilepsy, they may induce severe skin toxicity, potentially leading to fatal outcomes. Healthcare providers must remain vigilant for early symptoms and intervene promptly to mitigate adverse effects. This case report, alongside a literature review, aims to underscore the importance of comprehensive care throughout the preconception, pregnancy, and postpartum phases for women with epilepsy.
Keywords: Case report; Epilepsy; Lamotrigine; Literature review; Planning pregnancy; Stevens-Johnson syndrome; Toxic epidermal necrolysis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. It has been reviewed and approved by the Research Ethics Committee of the Beijing University of Chinese Medicine Third Affiliated Hospital (BZYSY-2024YJSKTPJ-13). Consent for publication: Written informed consent was obtained from the patient for publication of this case report. Competing interests: The authors declare no competing interests.
Figures
References
-
- Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol. 2018;54(1):147–76. - PubMed
-
- Viale L, Allotey J, Cheong-See F, Arroyo-Manzano D, McCorry D, Bagary M, Mignini L, Khan KS, Zamora J, Thangaratinam S, et al. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386(10006):1845–52. - PubMed
-
- Wang ML, Tao YY, Sun XY, Guo Y, Wang ZY, Cao YF, Zhao L. Estrogen profile- and pharmacogenetics-based lamotrigine dosing regimen optimization: recommendations for pregnant women with epilepsy. Pharmacol Res. 2021;169:105610. - PubMed
-
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, Sabers A, Thomas SV, Vajda F. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Letter to National Traditional Chinese Medicine Educators [2022] No. 1/National Administration of Traditional Chinese Medicine National Clinical Excellent Talents Advanced Study and Training Program
- Y2023A05/Beijing University of Chinese Medicine 'Qihuang Elite Talent · Famous Doctor Cultivation Program'
LinkOut - more resources
Full Text Sources
Medical

