Harnessing Dye-Induced Local Heating in Lipid Membranes: A Path to Near-Infrared Light-Modulated Artificial Synaptic Vesicles
- PMID: 40717382
- PMCID: PMC12367230
- DOI: 10.1021/acsnano.5c08482
Harnessing Dye-Induced Local Heating in Lipid Membranes: A Path to Near-Infrared Light-Modulated Artificial Synaptic Vesicles
Abstract
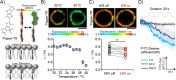
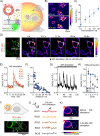
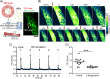
Optical heating coupled with near-infrared (NIR) light and photothermal materials enables local heating within biospecimens, minimizing undesirable thermal damage. Here, we demonstrated that photothermally heating lipid bilayers embedded with a phthalocyanine dye (VPc) efficiently perturbs the bilayers, resulting in increased permeability. Notably, microscopic studies revealed that the changes in membrane permeability may not follow the conventional mechanism of temperature-sensitive liposomes, which involve a bulk temperature increase that induces a phase transition across the entire lipid bilayer. Furthermore, the heat generated by NIR laser illumination rarely diffused into the surrounding environment, and the dye was located within the bilayers at the molecular level, where it effectively transferred heat to the lipid bilayer. We prepared VPc-embedded liposomes encapsulating acetylcholine (ACh) and demonstrated the NIR laser-triggered release of ACh, creating a concentration jump across a few cells or within a limited single cell region. This method induced Ca2+ flux through ACh receptor stimulation in thermally delicate biospecimens such as C2C12 myotubes and the Drosophila brain.
Keywords: NIR light; artificial synaptic vesicle; local heating; photothermal dye; thermometry.
Figures






Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Serotonin Promotes Vesicular Association and Fusion by Modifying Lipid Bilayers.J Phys Chem B. 2024 May 23;128(20):4975-4985. doi: 10.1021/acs.jpcb.4c00115. Epub 2024 May 14. J Phys Chem B. 2024. PMID: 38743687
-
Thermal-controlled cellular uptake of "hot" nanoparticles.Nanoscale. 2023 Aug 3;15(30):12718-12727. doi: 10.1039/d3nr02449k. Nanoscale. 2023. PMID: 37470374
-
Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults.Cochrane Database Syst Rev. 2016 Apr 21;4(4):CD009016. doi: 10.1002/14651858.CD009016.pub2. Cochrane Database Syst Rev. 2016. PMID: 27098439 Free PMC article.
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
Cited by
-
A Succinoglycan-Riclin-Zinc-Phthalocyanine-Based Composite Hydrogel with Enhanced Photosensitive and Antibacterial Activity Targeting Biofilms.Gels. 2025 Aug 21;11(8):672. doi: 10.3390/gels11080672. Gels. 2025. PMID: 40868802 Free PMC article.
References
-
- Gu Y., Piñol R., Moreno-Loshuertos R., Brites C. D. S., Zeler J., Martínez A., Maurin-Pasturel G., Fernández-Silva P., Marco-Brualla J., Téllez P., Cases R., Belsué R. N., Bonvin D., Carlos L. D., Millán A.. Local Temperature Increments and Induced Cell Death in Intracellular Magnetic Hyperthermia. ACS Nano. 2023;17(7):6822–6832. doi: 10.1021/acsnano.3c00388. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

