Dietary cholesterol impairs cognition via gut microbiota-derived deoxycholic acid in obese mice
- PMID: 40717663
- PMCID: PMC12309536
- DOI: 10.1080/19490976.2025.2537753
Dietary cholesterol impairs cognition via gut microbiota-derived deoxycholic acid in obese mice
Abstract
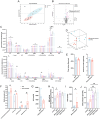
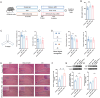
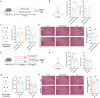
Dietary cholesterol is often found in a high-fat diet (HFD) and excessive intake is harmful to cognitive function. The gut microbiome constitutes an environmental factor influenced by diet, which regulates cognitive function via the gut-brain axis. The present study explored the role of dietary cholesterol in HFD-induced cognitive impairment and the participation of the gut microbiota and metabolites. Here, we found that dietary cholesterol promoted cognitive impairment in HFD-fed mice, which was associated with an increase in gut microbiota containing 7α-dehydroxylase, including Lachnospiraceae bacterium, Dorea sp. Clostridium sp. and elevated levels of deoxycholic acid (DCA) in the hippocampus. Upon dietary cholesterol intake, the activity of gut microbiota in mice to produce DCA is increased. Fecal microbiota transplantation confirmed that the cognitive impairment-promoting process was driven by gut microbiota. Reducing circulating bile acid levels with cholestyramine improved cognitive decline in mice, whereas hippocampal administration of DCA worsened cognitive function. Pharmacological inhibition of hippocampal apical sodium bile acid transporter reduces neuronal DCA accumulation and improves neuronal apoptosis as well as cognitive impairments in mice. Overall, this study revealed that dietary cholesterol promotes HFD-induced cognitive impairment by inducing the production of DCA through gut microbiota metabolism.
Keywords: Dietary cholesterol; bile acid; cognitive impairment; deoxycholic acid; gut microbiota.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning, H, Carnethon, MR, Greenland P, Mentz RJ, Tucker KL, Zhao L, et al. Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. J Sport Hist American Medical Association. 2019;321(11):1081–1095. doi: 10.1001/jama.2019.1572. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
