Molar Mass Improves the Performance of n‑Type Organic Electrochemical Transistors
- PMID: 40717785
- PMCID: PMC12288006
- DOI: 10.1021/acs.chemmater.5c00949
Molar Mass Improves the Performance of n‑Type Organic Electrochemical Transistors
Abstract
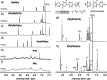
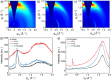
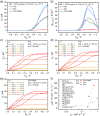
We report on the synthesis and use of two side-chain-free ionenes with varying heteroatoms, PFu and PTh, for n-type accumulation-mode OECTs. Compared to PTh, PFu is more challenging to make, less stable, and shows increased water solubility. The optical properties and surface morphologies of the two derivatives are comparable, but their microstructures vary distinctly in terms of ordering and backbone orientation. While the backbones of PTh show a preferential face-on orientation, PFu is significantly less ordered. The OECT performance of PTh is improved by 1 order of magnitude compared to PFu, as indicated by μC* values of 116.16 and 10.66 F cm-1 V-1 s-1, respectively. Further increasing the molar mass of PTh doubles the performance, resulting in a record-high μC* value of 225.71 F cm-1 V-1 s-1 and a high μ value of 0.58 cm2 V-1 s-1, highlighting the crucial role of molecular weight control for enhancing device performance.
© 2025 The Authors. Published by American Chemical Society.
Figures


Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Influence of Interaction between Electrolyte with Side-Chain Free Conjugated Polymer on the Performance of Organic Electrochemical Transistors.ACS Appl Mater Interfaces. 2024 Apr 24;16(16):19977-19986. doi: 10.1021/acsami.3c13781. Epub 2023 Dec 14. ACS Appl Mater Interfaces. 2024. PMID: 38096430 Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Novel application of metabolic imaging of early embryos using a light-sheet on-a-chip device: a proof-of-concept study.Hum Reprod. 2025 Jan 1;40(1):41-55. doi: 10.1093/humrep/deae249. Hum Reprod. 2025. PMID: 39521726 Free PMC article.
-
Pharmacological treatment of children with gastro-oesophageal reflux.Cochrane Database Syst Rev. 2014 Nov 24;2014(11):CD008550. doi: 10.1002/14651858.CD008550.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2023 Aug 22;8:CD008550. doi: 10.1002/14651858.CD008550.pub3. PMID: 25419906 Free PMC article. Updated.
References
-
- Rivnay J., Inal S., Salleo A., Owens R. M., Berggren M., Malliaras G. G.. Organic Electrochemical Transistors. Nat. Rev. Mater. 2018;3(2):17086. doi: 10.1038/natrevmats.2017.86. - DOI
-
- Tian Z., Zhao Z., Yan F.. Organic Electrochemical Transistor in Wearable Bioelectronics: Profiles, Applications, and Integration. Wearable Electron. 2024;1:1–25. doi: 10.1016/j.wees.2024.03.002. - DOI
-
- van de Burgt Y., Lubberman E., Fuller E. J., Keene S. T., Faria G. C., Agarwal S., Marinella M. J., Alec Talin A., Salleo A.. A Non-Volatile Organic Electrochemical Device as a Low-Voltage Artificial Synapse for Neuromorphic Computing. Nat. Mater. 2017;16(4):414–418. doi: 10.1038/nmat4856. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
