Intranasal delivery of dodecyl creatine ester alleviates motor deficits and increases dopamine levels in a 6-OHDA rat model of parkinsonism
- PMID: 40717895
- PMCID: PMC12289593
- DOI: 10.3389/fnagi.2025.1597263
Intranasal delivery of dodecyl creatine ester alleviates motor deficits and increases dopamine levels in a 6-OHDA rat model of parkinsonism
Abstract
Introduction: Creatine has been recognized not only as an energy buffer but also for its antioxidant, antiapoptotic, and anti-excitotoxic properties, making it of interest as a neuroprotective agent. Oral creatine monohydrate supplementation is ineffective due to poor brain and neuronal distribution and optimized forms of creatine deserve to be studied. Thus, dodecyl creatine ester (DCE), named CBT101, is a prodrug of creatine created for this purpose. When administered nasally it can follow the nose-to-brain pathway to deliver creatine to neuronal cells after passive diffusion across membranes. In this study, the therapeutic efficacy of intranasal DCE treatment was demonstrated in a 6-OHDA-intoxicated rat model, which is relevant to neurodegenerative diseases such as Parkinson's disease.
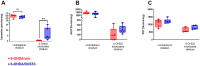
Methods: 6-OHDA-intoxicated rats received DCE (13.3 mg/kg/day) or a vehicle intranasally for 5 weeks and were compared to a sham group. Imbalance in dopamine between the two hemispheres was assessed using the amphetamine-induced turning test after 3 weeks and sensorimotor performance using the beam walking test after 4 weeks, with ongoing treatment.
Results and discussion: Five weeks after 6-OHDA intoxication, daily intranasal DCE treatment improved sensorimotor performance, striatal dopamine concentration, and modulated striatal pro-BDNF/BDNF balance and neurofilament expression both in plasma and in the striatum. These observations highlight DCE's potential as a therapeutic strategy for neurodegenerative diseases characterized by energy deficiency and major mitochondrial dysfunction.
Keywords: 6-hydroxydopamine; Parkinson’s disease; dodecyl creatine ester; intranasal drug delivery; mitochondrial dysfunction; motor behavior.
Copyright © 2025 Disdier, Lhotellier, Wagner, Andriambeloson, Théodoro, Pruvost, Joudinaud, Bénech and Mabondzo.
Conflict of interest statement
Dodecyl creatine ester or DCE (also named CBT101) is being developed by Ceres Brain Therapeutics. CD was an employee of Ceres Brain Therapeutics and is a shareholder of the company. CL is an employee of Ceres Brain Therapeutics. TJ is a co-founder and consultant of Ceres Brain Therapeutics. HB is an employee and co-founder of Ceres Brain Therapeutics. AM is a co-founder and a consultant of Ceres Brain Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Ahmadian N., Mahmoudi J., Talebi M., Molavi L., Sadigh-Eteghad S., Rostrup E., et al. (2018). Sleep deprivation disrupts striatal anti-apoptotic responses in 6-hydroxy dopamine-lesioned parkinsonian rats. Iran. J. Basic Med. Sci. 21, 1289–1296. doi: 10.22038/ijbms.2018.28546.6919, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

