Occult tethered cord syndrome: insights into clinical and MRI features, prognostic factors, and treatment outcomes in 30 dogs with confirmed or presumptive diagnosis
- PMID: 40717908
- PMCID: PMC12290461
- DOI: 10.3389/fvets.2025.1588538
Occult tethered cord syndrome: insights into clinical and MRI features, prognostic factors, and treatment outcomes in 30 dogs with confirmed or presumptive diagnosis
Abstract
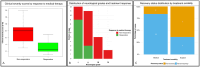
Occult tethered cord syndrome (OTCS) is poorly documented in dogs. This retrospective multicenter study evaluated the clinical presentation, MRI findings, treatment outcomes, and prognostic factors in 30 dogs diagnosed with OTCS managed surgically (n = 11) or medically (n = 19). Novel clinical severity scoring and neurological grading systems were developed to assess prognostic utility. The median age at clinical onset was 11 months (range 2-65), with a median duration of clinical signs of 13 months (range 1-60). Pain/dysesthesia in the lumbosacral region/tail/pelvic limbs was the most common presenting complaint (97%), followed by pelvic limb gait abnormalities (70%), behavioral changes (67%), impaired physical activity (63%), and urinary/fecal incontinence (17%). Neurological deficits were present in 90% of dogs. MRI findings showed variability in conus medullaris and dural sac termination, with no physiological translocation detected in available dynamic studies. Electrodiagnostic abnormalities were identified in four of nine tested dogs (44%). Clinical severity scores strongly predicted response to medical treatment, with responders having significantly lower scores than non-responders (3.25 ± 2.09 vs. 7.78 ± 3.15, p < 0.001). Higher neurological grades (p = 0.006), presence of behavioral abnormalities (p = 0.045), and worsening clinical evolution prior to referral (p = 0.009) were also associated with poor medical therapy outcomes. Surgical intervention was significantly associated with full recovery (p = 0.015) and discontinuation of medical treatment (p = 0.023) at last follow-up (median: 9 months, range: 2-108). Three surgically treated dogs experienced partial relapse within 6 months, with two undergoing reintervention and improving postoperatively. This study highlights the clinical and MRI characteristics of canine OTCS, introduces novel prognostic factors, and supports surgical detethering as a key intervention for optimizing outcomes. Larger prospective studies are needed to validate these findings, refine the proposed scoring systems, and establish evidence-based guidelines for managing canine OTCS.
Keywords: behavior; conus medullaris; dural sac; dynamic MRI; filum terminale; incontinence; lumbosacral; pain.
Copyright © 2025 Espinosa Romero, De Decker, Santifort, Gutierrez-Quintana, Ortega, Uriarte, Mojarradi, van Koulil, Douralidou, Espadas, Benito Benito, Anselmi, Dye, Alvarez, Minguez, Crawford and Posporis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer VL declared a past collaboration with the author JM to the handling editor.
Figures




Similar articles
-
Does Augmenting Irradiated Autografts With Free Vascularized Fibula Graft in Patients With Bone Loss From a Malignant Tumor Achieve Union, Function, and Complication Rate Comparably to Patients Without Bone Loss and Augmentation When Reconstructing Intercalary Resections in the Lower Extremity?Clin Orthop Relat Res. 2025 Jun 26. doi: 10.1097/CORR.0000000000003599. Online ahead of print. Clin Orthop Relat Res. 2025. PMID: 40569278
-
Effect of untethering on occult tethered cord syndrome: a systematic review.Br J Neurosurg. 2022 Oct;36(5):574-582. doi: 10.1080/02688697.2021.1995589. Epub 2021 Oct 28. Br J Neurosurg. 2022. PMID: 34709093
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Medical and surgical interventions for the treatment of usual-type vulval intraepithelial neoplasia.Cochrane Database Syst Rev. 2016 Jan 5;2016(1):CD011837. doi: 10.1002/14651858.CD011837.pub2. Cochrane Database Syst Rev. 2016. PMID: 26728940 Free PMC article.
Cited by
-
Anatomical and histological characterization of the filum terminale in dogs.Front Vet Sci. 2025 Jul 24;12:1650893. doi: 10.3389/fvets.2025.1650893. eCollection 2025. Front Vet Sci. 2025. PMID: 40777825 Free PMC article.
References
LinkOut - more resources
Full Text Sources

