Lumefantrine ameliorates DSS-induced colitis by targeting FLI-1 to suppress NF-κB signaling
- PMID: 40717962
- PMCID: PMC12289662
- DOI: 10.3389/fphar.2025.1614978
Lumefantrine ameliorates DSS-induced colitis by targeting FLI-1 to suppress NF-κB signaling
Abstract
Background: Current therapeutic options for inflammatory bowel disease (IBD) remain suboptimal due to limited efficacy, significant side effects, and high relapse rates, necessitating novel treatment strategies. Lumefantrine, a clinically established antimalarial drug, emerges as a compelling repurposing candidate based on its putative anti-inflammatory activity, though its efficacy and mechanism in IBD remain unexplored.
Methods: A murine IBD model was induced by 3% dextran sulfate sodium (DSS). Mice received oral Lumefantrine (20 mg/kg/day) for 7 days. Disease progression was monitored via disease activity index (DAI) scoring and histological analysis. Serum cytokines (IL-1β, IL-6, TNF-α) and colonic inflammatory mediators (Cox-2, iNos) were quantified by ELISA and qPCR. Tight junction proteins (Claudin-1, ZO-1) were assessed by immunohistochemistry and Western blot. Molecular targets were identified through computational docking and pull-down assays. Additionally, NF-κB signaling modulation was assessed in lipopolysaccharide (LPS)-stimulated intestinal epithelial cells (IEC-6 and NCM460) via Western blot analysis.
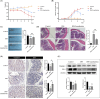
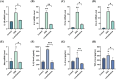
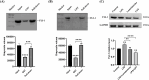
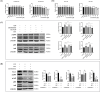
Results: Oral administration of Lumefantrine significantly attenuated disease activity index (DAI) scores and restored intestinal barrier integrity through upregulation of epithelial tight junction proteins Claudin-1 and ZO-1. Treated mice exhibited reduced serum levels of IL-1β, IL-6 and TNF-α, along with decreased colonic expression of inflammatory mediators cyclooxygenase-2 (Cox-2) and inducible nitric oxide synthase (iNos). Computational and experimental approaches identified FLI-1 a transcription factor upregulated in IBD colon tissues as Lumefantrine's direct binding target. This interaction mediated suppression of NF-κB signaling, specifically downregulating phosphorylation of IκBα and p65 in LPS-stimulated intestinal epithelial cells.
Conclusion: Lumefantrine ameliorates experimental colitis through FLI-1-dependent inhibition of the NF-κB pathway, demonstrating high repurposing potential as an IBD therapeutic.
Keywords: Fli-1; Lumefantrine; NF-κB; colitis; inflammatory bowel disease (IBD).
Copyright © 2025 Yang, Guo, Luo, Tang, Liu and Ren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Anti-colitic effects of Oldenlandia umbellata L. through NF-κB pathway inhibition in a DSS-induced mouse model.Inflammopharmacology. 2025 Jul;33(7):4037-4050. doi: 10.1007/s10787-025-01808-9. Epub 2025 Jun 23. Inflammopharmacology. 2025. PMID: 40549321
-
Network pharmacology and experimental validation reveal Thiamphenicol ameliorates dextran sulfate sodium-induced colitis in mice via inhibition of intestinal senescence and modulation of the NF-κB/AMPK signaling pathway.Toxicol Appl Pharmacol. 2025 Oct;503:117497. doi: 10.1016/j.taap.2025.117497. Epub 2025 Jul 30. Toxicol Appl Pharmacol. 2025. PMID: 40749954
-
Exploring the beneficial effects of GHK-Cu on an experimental model of colitis and the underlying mechanisms.Front Pharmacol. 2025 Jul 2;16:1551843. doi: 10.3389/fphar.2025.1551843. eCollection 2025. Front Pharmacol. 2025. PMID: 40672369 Free PMC article.
-
Fucoidan as a therapeutic agent for ulcerative colitis: mechanisms of action and modulation of the gut microbiota.Front Cell Infect Microbiol. 2025 Jul 10;15:1626614. doi: 10.3389/fcimb.2025.1626614. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40708752 Free PMC article. Review.
-
Tolerability of selective cyclooxygenase 2 inhibitors used for the treatment of rheumatological manifestations of inflammatory bowel disease.Cochrane Database Syst Rev. 2014 Oct 23;2014(10):CD007744. doi: 10.1002/14651858.CD007744.pub2. Cochrane Database Syst Rev. 2014. PMID: 25340915 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

