Harnessing Antiviral Peptides: From Molecular Mechanisms to Clinical Translation
- PMID: 40718095
- PMCID: PMC12296541
- DOI: 10.1016/j.crphar.2025.100228
Harnessing Antiviral Peptides: From Molecular Mechanisms to Clinical Translation
Abstract
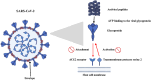
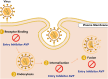
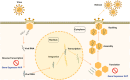
Viral infections continue to pose a significant threat to global health, especially with the emergence and re-emergence of resistant viral strains. The limitations of conventional antiviral therapies, such as narrow-spectrum activity, high toxicity, and rising resistance, underscore the need for innovative treatment strategies. Antiviral peptides (AVPs) have gained attention as promising therapeutic agents due to their broad-spectrum antiviral activity, low cytotoxicity, and ability to target multiple stages of the viral life cycle. This review provides a comprehensive overview of AVPs, focusing on their classification, mechanisms of action, and clinical relevance. Both natural and synthetic AVPs are discussed, including FDA-approved agents such as enfuvirtide (HIV) and boceprevir (HCV), along with candidates currently in clinical trials. AVPs inhibit viral attachment, fusion, replication, and assembly, while also modulating host immune responses. Their applications extend beyond treatment to include prophylaxis and combination therapies, offering potential benefits in pandemic preparedness. However, challenges such as enzymatic degradation, poor bioavailability, and high production costs limit their clinical translation. Recent advances in peptide engineering, computational drug design, and nanoparticle-based delivery systems aim to overcome these barriers. AVPs represent a promising class of antiviral agents with the potential to address current therapeutic gaps and improve future outbreak response. This review highlights their growing importance in the field of antiviral therapy and outlines future directions for research and development.
Keywords: Antiviral peptides; Broad spectrum; Entry inhibitor; Enveloped; Pandemic preparedness; Prophylaxis.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Deep Generative Models for the Discovery of Antiviral Peptides Targeting Dengue Virus: A Systematic Review.Int J Mol Sci. 2025 Jun 26;26(13):6159. doi: 10.3390/ijms26136159. Int J Mol Sci. 2025. PMID: 40649934 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Deep learning in the discovery of antiviral peptides and peptidomimetics: databases and prediction tools.Mol Divers. 2025 Aug;29(4):3753-3788. doi: 10.1007/s11030-025-11173-y. Epub 2025 Mar 28. Mol Divers. 2025. PMID: 40153158 Review.
References
-
- Amin A., Zaccardi J., Mullen S., Olland S., Orlowski M., Feld B., Labonte P., Mak P. Identification of constrained peptides that bind to and preferentially inhibit the activity of the hepatitis C viral RNA-dependent RNA polymerase. Virology. 2003;313:158–169. doi: 10.1016/S0042-6822(03)00313-1. - DOI - PubMed
-
- Ashaolu T.J., Nawaz A., Walayat N., Khalifa I. Potential “biopeptidal” therapeutics for severe respiratory syndrome coronaviruses: a review of antiviral peptides, viral mechanisms, and prospective needs. Appl. Microbiol. Biotechnol. 2021;105:3457–3470. doi: 10.1007/s00253-021-11267-1. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

