Open or closed? Understanding the molecular mechanisms and clinical implications of ADAMTS13's conformation
- PMID: 40718105
- PMCID: PMC12296723
- DOI: 10.1002/hem3.70189
Open or closed? Understanding the molecular mechanisms and clinical implications of ADAMTS13's conformation
Abstract
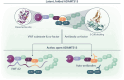
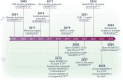
By proteolyzing prothrombotic von Willebrand factor (VWF) multimers, ADAMTS13 (A Disintegrin And Metalloproteinase with ThromboSpondin type-1 repeats, member 13) ensures balanced hemostasis and prevents microvascular thrombosis. ADAMTS13's conformational regulation is not only crucial for its enzymatic function, but also for the pathophysiology of thrombotic thrombocytopenic purpura (TTP). In the first part of this review, the unique structural features that keep ADAMTS13 in its closed, latent conformation are explored. Moreover, the recent structure predictions that propose a compactly folded model for closed ADAMTS13, and the molecular mechanisms involved in ADAMTS13's opening by its VWF substrate and other allosteric activators are discussed. Over the last decade, the changes in ADAMTS13's conformation in the context of immune-mediated TTP (iTTP) were increasingly characterized, with open ADAMTS13 having emerged as a novel specific biomarker for acute and subclinical iTTP. Furthermore, open ADAMTS13 is gaining clinical attention to improve the prediction of early relapses during follow-up of iTTP patients in remission. The specificity of the open ADAMTS13 biomarker for iTTP was retrospectively validated in patient cohorts with various thrombotic microangiopathies or hemostatic disorders, all dominantly presenting closed ADAMTS13. Hence, this review summarizes the molecular mechanisms that regulate ADAMTS13's conformation and links these with the clinical implications of ADAMTS13's open and closed conformations.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
[100 years thrombotic thrombocytopenic purpura (TTP) - lessons learned?].Dtsch Med Wochenschr. 2024 Nov;149(23):1423-1430. doi: 10.1055/a-2360-8725. Epub 2024 Nov 6. Dtsch Med Wochenschr. 2024. PMID: 39504978 German.
-
Immune thrombotic thrombocytopenic purpura: pathogenesis and novel therapies: a narrative review.Ann Blood. 2023 Sep 30;8:26. doi: 10.21037/aob-22-29. Epub 2023 Jan 6. Ann Blood. 2023. PMID: 39100389 Free PMC article.
-
Plasma Levels of Big Endothelin-1 Are Associated with Renal Insufficiency and In-Hospital Mortality of Immune Thrombotic Thrombocytopenic Purpura.Thromb Haemost. 2022 Mar;122(3):344-352. doi: 10.1055/a-1508-8347. Epub 2021 Jun 21. Thromb Haemost. 2022. PMID: 33984867 Free PMC article.
-
Laboratory Testing for ADAMTS13 for Thrombotic Thrombocytopenia Purpura and Beyond.Semin Thromb Hemost. 2025 Sep;51(6):687-697. doi: 10.1055/s-0044-1792003. Epub 2024 Oct 28. Semin Thromb Hemost. 2025. PMID: 39467573 Review.
-
Thrombotic thrombocytopenic purpura: early diagnosis and effective treatment in 2025.Intensive Care Med. 2025 Jul;51(7):1225-1239. doi: 10.1007/s00134-025-07981-3. Epub 2025 Jul 3. Intensive Care Med. 2025. PMID: 40608084 Review.
References
-
- Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413(6855):488‐494. - PubMed
-
- Gerritsen HE, Robles R, Lämmle B, Furlan M. Partial amino acid sequence of purified von Willebrand factor–cleaving protease. Blood. 2001;98(6):1654‐1661. - PubMed
-
- Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand Factor‐cleaving Protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276(44):41059‐41063. - PubMed
-
- Soejima K, Mimura N, Hiroshima M, et al. A novel human metalloprotease synthesized in the liver and secreted into the blood: possibly, the von willebrand factor‐cleaving protease? J Biochem. 2001;130(4):475‐480. - PubMed
-
- Fujikawa K, Suzuki H, McMullen B, Chung D. Purification of human von Willebrand factor–cleaving protease and its identification as a new member of the metalloproteinase family. Blood. 2001;98(6):1662‐1666. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
