Alkaline phosphatase-streptavidin conjugate (APSA) enzyme and binding activity over time and storage conditions
- PMID: 40718384
- PMCID: PMC12296546
- DOI: 10.1016/j.bbrep.2025.102160
Alkaline phosphatase-streptavidin conjugate (APSA) enzyme and binding activity over time and storage conditions
Abstract
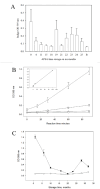
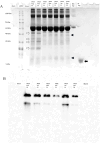
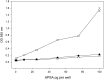
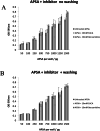
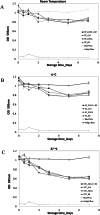
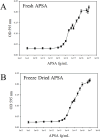
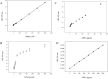
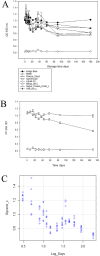
Alkaline phosphatase (AP) linked to streptavidin (SA) in the form of the APSA enzyme conjugate is required for diagnostic screening for a variety of clinical conditions world wide. The enzyme activity of APSA conjugates in the liquid phase showed variation across samples that declined with storage time. Random sampling of the enzyme activity in the liquid phase (ANOVA p < 2E-16; Regression p < 0.043) and the binding plus enzyme activity of APSA in the model assay (R2 > 0.99) of biotinylated human IgG (B-h-IgG) directly adsorbed to 96 well plates showed a similar loss of function over time (ANOVA p < 9.15E-15, Regression p <1.1E-9). The enzyme AP showed little dissociation from the SA moiety while proteolysis of the BSA carrier was observed. Covalent protease inhibitors 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF) or tosyl-l-lysine chloromethyl ketone hydrochloride (TLCK) abrogated AP enzyme activity, but the competitive inhibitors epsilon-aminocaproic acid (EACA) and benzamidine (BNZ) had no protective effect on APSA activity over time. Samples of APSA showed large variation in enzyme activity (p ≤ 2E-16) and so were titrated by the colorimetric assay and standardized against indigo blue in DMSO to achieve an initial OD value of ∼1.0 at 595 nm prior to following activity over storage time. After titration, the effect of temperature, addition of glycerol prior to freezing, and freeze drying with or without trehalose and sucrose, on alkaline phosphatase activity was compared using a sampling schedule over storage time. The alkaline phosphate activity was not immediately sensitive to freeze-drying but was sensitive to storage time and ultra-low temperatures, but the addition of sugars or glycerol to the APSA prevented some of the activity loss. Storage of APSA on wet ice or in 50 % glycerol at -20 °C retained about 50 % of the starting optical density reading of APSA after 170 days in storage.
Keywords: APSA, alkaline phosphatase streptavidin conjugate; BCIP/NBT; Freezing, freeze drying; Glycerol, sucrose, trehalose; Protease inhibitors; Storage conditions.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Thermal stability and storage of human insulin.Cochrane Database Syst Rev. 2023 Nov 6;11(11):CD015385. doi: 10.1002/14651858.CD015385.pub2. Cochrane Database Syst Rev. 2023. PMID: 37930742 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
References
-
- Blake M.S., et al. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal. Biochem. 1984;136(1):175–179. - PubMed
-
- Cook D.B., Self C.H. Determination of one thousandth of an attomole (1 zeptomole) of alkaline phosphatase: application in an immunoassay of proinsulin. Clin. Chem. 1993;39(6):965–971. - PubMed
-
- Simopoulos T.T., Jencks W.P. Alkaline phosphatase is an almost perfect enzyme. Biochemistry. 1994;33(34):10375–10380. - PubMed
-
- Self C.H. Enzyme amplification--a general method applied to provide an immunoassisted assay for placental alkaline phosphatase. J. Immunol. Methods. 1985;76(2):389–393. - PubMed
LinkOut - more resources
Full Text Sources

