Trichinella spiralis-derived extracellular vesicles induce regulatory T cells and reduce airway allergy in mice
- PMID: 40718495
- PMCID: PMC12289487
- DOI: 10.3389/fimmu.2025.1637569
Trichinella spiralis-derived extracellular vesicles induce regulatory T cells and reduce airway allergy in mice
Abstract
Introduction: Respiratory allergies are an increasing global health concern, with current treatments primarily targeting symptoms rather than underlying immune dysregulation. Trichinella spiralis-derived extracellular vesicles (TsEVs) have been implicated in modulating immune responses, but their role in allergic airway inflammation remains unexplored. This study investigates the immunomodulatory potential of TsEVs in mitigating ovalbumin (OVA)-induced allergic airway inflammation in mice.
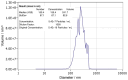
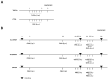
Methods: TsEVs were isolated from T. spiralis muscle larvae excretory-secretory products and characterized using nanoparticle tracking analysis. BALB/c mice were sensitized and challenged intranasally with OVA to induce respiratory allergy. TsEVs were administered intranasally before and during OVA challenge. Bronchoalveolar lavage fluid (BALF), lung tissue, spleens, and sera were analyzed for immune cell infiltration, cytokine production, regulatory T cell (Treg) expansion, and OVA-specific antibodies using histology, flow cytometry, and ELISA.
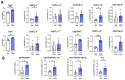
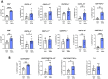
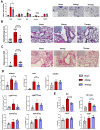
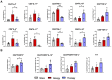
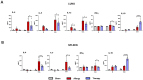
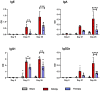
Results: Intranasal administration of TsEVs significantly reduced eosinophilic infiltration and airway inflammation in OVA-sensitized mice. TsEVs treatment suppressed Th2 cytokines (IL-4, IL-5, IL-13) and OVA-specific IgE while enhancing IL-10 production. Importantly, TsEVs promoted expansion of CD4+FoxP3+ and CD4+FoxP3-IL-10+ regulatory T cells in lungs and spleen, contributing to a systemic anti-inflammatory profile. Ex vivo studies confirmed TsEVs-mediated modulation of allergen-stimulated immune responses.
Discussion: Our findings highlight TsEVs as a promising therapeutic approach for allergic airway diseases by promoting immune tolerance and dampening inflammatory responses. These results pave the way for future translational applications of parasite-derived EVs in allergy treatment.
Keywords: Trichinella spiralis; allergic inflammation; extracellular vesicles; immune modulation; regulatory T cells; respiratory allergy.
Copyright © 2025 Glamočlija, Sabljić, Schmid, Radulović, Gruden-Movsesijan, Vasilev, Inić-Kanada, Wiedermann, Schabussova and Kosanović.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Trichinella spiralis adult excretory-secretory antigen promotes peripheral regulatory T cell differentiation and attenuates experimental colitis via TGF-β-like mechanisms.Parasit Vectors. 2025 Jul 1;18(1):240. doi: 10.1186/s13071-025-06877-x. Parasit Vectors. 2025. PMID: 40597393 Free PMC article.
-
Trichinella spiralis excretory-secretory protein alleviates autoimmune thyroiditis by modulating Th17/Treg balance via the STAT3/STAT5 pathway.Acta Trop. 2025 Aug;268:107706. doi: 10.1016/j.actatropica.2025.107706. Epub 2025 Jun 23. Acta Trop. 2025. PMID: 40562184
-
Protection against allergic airway inflammation during the chronic and acute phases of Trichinella spiralis infection.Clin Exp Allergy. 2013 Jan;43(1):103-15. doi: 10.1111/cea.12042. Clin Exp Allergy. 2013. PMID: 23278885
-
The Role of Extracellular Vesicles in Allergic Sensitization: A Systematic Review.Int J Mol Sci. 2024 Apr 19;25(8):4492. doi: 10.3390/ijms25084492. Int J Mol Sci. 2024. PMID: 38674077 Free PMC article.
-
The Role of Epithelial-Derived Extracellular Vesicles in Allergic Sensitisation: A Systematic Review.Int J Mol Sci. 2025 Jun 17;26(12):5791. doi: 10.3390/ijms26125791. Int J Mol Sci. 2025. PMID: 40565252 Free PMC article.
References
-
- Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss MS. WAO White Book on Allergy. WI, USA: World Allergy Organization (WAO), (2013). pp. 81–103.
-
- Aït-Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J, et al. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy. (2009) 64:123–48. doi: 10.1111/j.1398-9995.2008.01884.x, PMID: - DOI - PubMed
-
- Walker S, Khan-Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: Case-control study. J Allergy Clin Immunol. (2007) 120:381–7. doi: 10.1016/j.jaci.2007.03.034, PMID: - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

