First-line systemic therapy in patients with metastatic or locally advanced urothelial carcinoma: a systematic review and network meta-analysis of randomized controlled trials
- PMID: 40718544
- PMCID: PMC12290386
- DOI: 10.1177/17588359251357527
First-line systemic therapy in patients with metastatic or locally advanced urothelial carcinoma: a systematic review and network meta-analysis of randomized controlled trials
Abstract
Background: The emergence of immune checkpoint inhibitors and antibody-drug conjugates has revolutionized the first-line treatment landscape for locally advanced or metastatic urothelial carcinoma (la/mUC). However, the optimal treatment strategy remains uncertain.
Objectives: This network meta-analysis (NMA) aimed to evaluate the efficacy and safety of various first-line treatments for la/mUC.
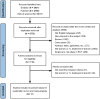
Design: Systematic literature review with a Bayesian NMA.
Data sources and methods: Eligible studies were retrieved from PubMed, EMBASE, and Web of Science, with a search cutoff of July 2024. Randomized controlled trials (RCTs) evaluating first-line treatments for la/mUC were included. Pairwise comparisons and Bayesian NMA were conducted to compare overall survival (OS) and progression-free survival (PFS) using hazard ratios (HR) and 95% credible intervals (CrIs), and objective response rate (ORR) and treatment-related adverse events (TRAEs) using odds ratios and 95% CrIs.
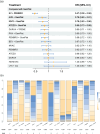
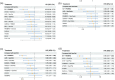
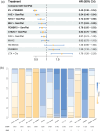
Results: In total, 17 articles involving 11 RCTs and 7586 patients were included. Enfortumab vedotin (EV) plus pembrolizumab demonstrated the most significant improvement in OS (HR 0.47, 95% CrI 0.38-0.58) compared to platinum-based chemotherapy in the overall populations, with consistent benefits across cisplatin-eligible, cisplatin-ineligible, and PD-L1-positive/negative subgroups. EV plus pembrolizumab also ranked highest for PFS (HR 0.45, 95% CrI 0.38-0.54) and had a favorable ORR compared to other regimens. In terms of safety, atezolizumab monotherapy exhibited the lowest incidence of high-grade TRAEs, EV plus pembrolizumab had higher overall TRAE rates but lower rates of grade 3 or higher TRAEs than platinum-based chemotherapy and nivolumab plus gemcitabine-cisplatin.
Conclusion: This NMA provides the most comprehensive analysis of first-line treatments for la/mUC, integrating the latest clinical data. EV plus pembrolizumab demonstrated superior efficacy and acceptable safety profiles in overall and subgroup analyses, establishing it as a promising treatment option.
Trial registration: The study was registered in PROSPERO (CRD42024502320).
Keywords: clinical trials; enfortumab vedotin; first-line treatment; network meta-analysis; survival outcomes; urothelial carcinoma.
Plain language summary
Comparing first-line treatments for advanced bladder and urinary tract cancer: Which drug combinations work best and safest? Why was the study done? Advanced bladder and urinary tract cancer is challenging to treat. While newer drugs like immunotherapy and antibody-based therapies have improved care, doctors still debate which first-line treatment is most effective and safest. This study aimed to compare all available treatments to identify the best options. What did the researchers do? The research team reviewed 17 publications reporting results from 11 randomized controlled trials involving 7,586 patients. The network meta-analysis was used to compare survival outcomes, response rates, and side effects of different drug combinations. Treatments included chemotherapy, immunotherapy, and newer combinations like enfortumab vedotin (EV) plus pembrolizumab. What did the researchers find? The combination of EV and pembrolizumab showed the best results. Patients receiving this combination lived longer (47% lower risk of death) and had better disease control compared to standard chemotherapy. These benefits were consistent across patients of different ages, health statuses, and tumor characteristics. While EV plus pembrolizumab caused more mild-to-moderate side effects (e.g., skin reactions), it had fewer severe side effects than chemotherapy. What do the findings mean? This study suggests that EV combined with pembrolizumab is currently the most effective first-line treatment for advanced bladder and urinary tract cancer, offering better survival and manageable side effects. These results support using this combination as a preferred option for many patients. The ranking of all available treatments also helps guide therapy selection when EV-based regimens are not suitable or accessible.
© The Author(s), 2025.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Lenvatinib plus pembrolizumab for untreated advanced renal cell carcinoma: a systematic review and cost-effectiveness analysis.Health Technol Assess. 2024 Aug;28(49):1-190. doi: 10.3310/TRRM4238. Health Technol Assess. 2024. PMID: 39252678 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Bellmunt J, Petrylak DP. New therapeutic challenges in advanced bladder cancer. Semin Oncol 2012; 39: 598–607. - PubMed
-
- von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005; 23: 4602–4608. - PubMed
-
- Logothetis CJ, Dexeus FH, Finn L, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol 1990; 8: 1050–1055. - PubMed
-
- Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1992; 10: 1066–1073. - PubMed
-
- Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 2006; 107: 506–513. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

