Heart Digital Twins Predict Features of Invasive Reentrant Circuits and Ablation Lesions in Scar-Dependent Ventricular Tachycardia
- PMID: 40718936
- PMCID: PMC12313252
- DOI: 10.1161/CIRCEP.124.013660
Heart Digital Twins Predict Features of Invasive Reentrant Circuits and Ablation Lesions in Scar-Dependent Ventricular Tachycardia
Abstract
Background: Catheter ablation of scar-dependent ventricular tachycardia (VT) is frequently hampered by hemodynamic instability, long procedure duration, and high recurrence rates. Magnetic resonance imaging-based personalized heart digital twins may overcome these challenges by noninvasively predicting VT circuits and optimum ablation lesion sites. In this combined clinical and digital twin study, we investigated the relationship between digital twin-predicted VTs and optimum ablation lesion sets with their invasively mapped counterparts during clinical VT ablation.
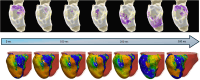
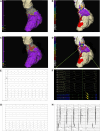
Methods: A total of 18 patients with scar-dependent VT underwent digital twin creation based on preprocedural, contrast-enhanced cardiac magnetic resonance imaging. Using rapid pacing protocols, VT was simulated and ablation targets were derived that would terminate all possible VTs in the models. Patients subsequently underwent invasive VT ablation, including targeting of diastolic activity and optimum entrainment sites. Digital twin-predicted VT circuits and ablation lesions were compared with their invasive clinical counterparts.
Results: Forty-three clinical VTs and 92 digital twin VTs were induced. Diastolic activity was seen in 16 of 43 (37.2%) clinical VTs. Sensitivity, specificity, positive predictive, and negative predictive values for the detection of critical VT sites by digital twins were 81.3%, 83.8%, 21.7%, and 98.8%, respectively. At an American Heart Association-segment level, agreement between clinical VT critical sites and digital twin primary predicted sites was moderate, with a κ coefficient of 0.46 (±0.32; P≤0.001). Termination of VT with ablation was achieved at a digital twin-predicted site in 4 of 5 (80%) cases where attempted. A total of 426 of 709 (60.1%) lesions were within 5 mm of a predicted target site. In total, 54.0% (±28.9%) of the digital twin-predicted area was ablated per patient based on conventional mapping criteria.
Conclusions: Heart digital twin VT circuits and ablation targets accurately predict many features of their respective clinical counterparts but have some limitations in spatial resolution. Our findings demonstrate the significant potential of digital twin technology in guiding catheter ablation for scar-dependent VT.
Keywords: catheter ablation; heart rate; humans; magnetic resonance imaging; technology.
Conflict of interest statement
None.
Figures



References
-
- Willems S, Tilz RR, Steven D, Kääb S, Wegscheider K, Gellér L, Meyer C, Heeger CH, Metzner A, Sinner MF, et al. ; BERLIN VT Investigators. Preventive or deferred ablation of ventricular tachycardia in patients with ischemic cardiomyopathy and implantable defibrillator (BERLIN VT): a multicenter randomized trial. Circulation. 2020;141:1057–1067. doi: 10.1161/CIRCULATIONAHA.119.043400 - PubMed
-
- Kuck KH, Tilz RR, Deneke T, Hoffmann BA, Ventura R, Hansen PS, Zarse M, Hohnloser SH, Kautzner J, Willems S; SMS Investigators. Impact of substrate modification by catheter ablation on implantable cardioverter-defibrillator interventions in patients with unstable ventricular arrhythmias and coronary artery disease: results from the multicenter randomized controlled SMS (Substrate Modification Study). Circ Arrhythm Electrophysiol. 2017;10:e004422. doi: 10.1161/CIRCEP.116.004422 - PubMed
-
- Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, Thibault B, Rivard L, Gula L, Leong-Sit P, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375:111–121. doi: 10.1056/NEJMoa1513614 - PubMed
-
- Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacrétaz E, Pitschner H-F, Kautzner J, Schumacher B, Hansen PS; VTACH Study Group. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet (London, England). 2010;375:31–40. doi: 10.1016/S0140-6736(09)61755-4 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

