A novel missense variant in TNFAIP3 associated with autoimmunity reveals the contribution of STAT1/mTOR pathways
- PMID: 40719110
- PMCID: PMC12457938
- DOI: 10.1093/cei/uxaf048
A novel missense variant in TNFAIP3 associated with autoimmunity reveals the contribution of STAT1/mTOR pathways
Abstract
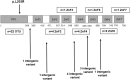
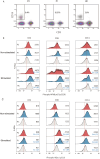
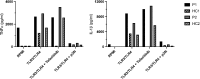
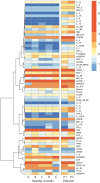
Heterozygous loss-of-function mutations in the TNFAIP3 gene lead to A20 haploinsufficiency (HA20). A20 protein is a negative feedback regulator of NF-κB signaling. Traditionally, HA20 is associated with Behçet's disease-like symptoms, however, recent findings suggest it may also manifest with a broader array of autoimmune diseases. Here, we describe a novel TNFAIP3 variant in a Dutch family, predominantly presenting with polyautoimmunity rather than autoinflammatory manifestations. We evaluated two patients from a Dutch family with autoimmune symptoms. Whole-exome sequencing (WES) identified a heterozygous c.608T>G (p.Leu203Arg) missense variant in TNFAIP3, located within the OTU domain. Functional analyses included immunoblotting of peripheral blood mononuclear cells (PBMCs) and an overexpression model using transfected HEK293T cells. A20 protein expression was evaluated, while phosphoflow cytometry assessed phosphorylation of key signaling molecules in the NF-κB, STAT and mTOR pathways. Inflammatory cytokine production was measured in cell culture supernatants. Overexpression of this missense A20 variant in HEK293T enhanced NF-κB signaling, reflected by increased TRAF6 expression and IκBα phosphorylation. Patient-derived PBMCs demonstrated reduced A20 expression, increased phosphorylation within the NF-κB, STAT1, and mTOR pathways, and elevated production of pro-inflammatory cytokines. These molecular alterations suggest disrupted immune regulation contributing to the observed autoimmune phenotype. The identification of this novel TNFAIP3 variant contributing to HA20 expands the clinical spectrum to include predominant autoimmune manifestations. In addition to NF-κB and STAT1 activation, we discovered mTOR pathway activation, shedding new light on A20's function and progression toward autoimmunity. Furthermore, the involvement of mTOR pathway also provides new therapeutic possibilities.
Keywords: A20 haploinsufficiency; NF-κB; STAT1 and mTOR pathway; TNFAIP3; polyautoimmunity.
© The Author(s) 2025. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
The authors declare no conflicts of interest. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors have no relevant financial or non-financial interests to disclose.
Figures




References
-
- Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet 2016, 48, 67–73. doi: https://doi.org/ 10.1038/ng.3459 - DOI - PMC - PubMed
-
- Shaheen ZR, Williams SJA, Binstadt BA.. Case report: a novel TNFAIP3 mutation causing haploinsufficiency of A20 with a lupus-like phenotype. Front Immunol 2021, 12, 629457. doi: https://doi.org/ 10.3389/fimmu.2021.629457 - DOI - PMC - PubMed
-
- Berteau F, Rouviere B, Nau A, Le Berre R, Sarrabay G, Touitou I, et al. ‘A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-kB-mediated autoinflammatory disease’. Ann Rheum Dis 2019, 78, e35. doi: https://doi.org/ 10.1136/annrheumdis-2018-213347 - DOI - PubMed
-
- Tsuchida N, Kirino Y, Soejima Y, Onodera M, Arai K, Tamura E, et al. Haploinsufficiency of A20 caused by a novel nonsense variant or entire deletion of TNFAIP3 is clinically distinct from Behcet’s disease. Arthritis Res Ther 2019, 21, 137. doi: https://doi.org/ 10.1186/s13075-019-1928-5 - DOI - PMC - PubMed
-
- Rossi MN, Federici S, Uva A, Passarelli C, Celani C, Caiello I, et al. Identification of a novel mutation in TNFAIP3 in a family with poly-autoimmunity. Front Immunol 2022, 13, 804401. doi: https://doi.org/ 10.3389/fimmu.2022.804401 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

