anti-Diastereo- and Enantioselective Ruthenium-Catalyzed C-C Coupling-Oxidative Lactonization of 1,4-Butanediol: Alkynes as Allylmetal Pronucleophiles
- PMID: 40719306
- PMCID: PMC12313187
- DOI: 10.1021/jacs.5c09358
anti-Diastereo- and Enantioselective Ruthenium-Catalyzed C-C Coupling-Oxidative Lactonization of 1,4-Butanediol: Alkynes as Allylmetal Pronucleophiles
Abstract
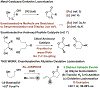
Reported methods for enantioselective oxidative lactonization affect desymmetrization of prochiral or meso-diols, and processes where C-C bond formation accompanies oxidative lactonization are undescribed. Here, using the ruthenium catalyst assembled from RuHCl(CO)(PPh3)3, JOSIPHOS SL-J009-1 and KI, allylative oxidative lactonizations of 1,4-butanediol (106 tons/yr) are described that occur with high levels of anti-diastereo- and enantioselectivity. As corroborated by deuterium labeling experiments, these processes merge three distinct catalytic events: (a) alkyne-to-allene isomerization, (b) transfer hydrogenative carbonyl (α-aryl)allylation of the allene pronucleophile, and (c) oxidative lactonization of the resulting enantiomerically enriched 1°,2°-diol.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
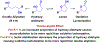


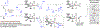
Similar articles
-
Diastereo- and Enantioselective Ruthenium-Catalyzed C-C Coupling of 1-Arylpropynes and Alcohols: Alkynes as Chiral Allylmetal Precursors in Carbonyl anti-(α-Aryl)allylation.J Am Chem Soc. 2021 Feb 24;143(7):2838-2845. doi: 10.1021/jacs.0c12242. Epub 2021 Feb 8. J Am Chem Soc. 2021. PMID: 33555867 Free PMC article.
-
Enantioselective ruthenium-catalyzed carbonyl allylation via alkyne-alcohol C-C bond-forming transfer hydrogenation: allene hydrometalation vs oxidative coupling.J Am Chem Soc. 2015 Mar 11;137(9):3161-4. doi: 10.1021/jacs.5b00747. Epub 2015 Mar 3. J Am Chem Soc. 2015. PMID: 25734220 Free PMC article.
-
Dual Ruthenium-Catalyzed Alkene Isomerization-Hydrogen Auto-Transfer Unlocks Skipped Dienes as Pronucleophiles for Enantioselective Alcohol C-H Allylation.J Am Chem Soc. 2023 Apr 5:10.1021/jacs.3c00934. doi: 10.1021/jacs.3c00934. Online ahead of print. J Am Chem Soc. 2023. PMID: 37018070 Free PMC article.
-
Antioxidants for male subfertility.Cochrane Database Syst Rev. 2014;(12):CD007411. doi: 10.1002/14651858.CD007411.pub3. Epub 2014 Dec 15. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Mar 14;3:CD007411. doi: 10.1002/14651858.CD007411.pub4. PMID: 25504418 Updated.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
References
-
-
For selected examples of ruthenium-catalyzed oxidative esterification of primary alcohols, see:
- Blum Y; Reshef D; Shvo Y H-Transfer Catalysis with Ru3(CO)12. Tetrahedron Lett. 1981, 22, 1541–1544.
- Zhang J; Leitus G; Ben-David Y; Milstein D Facile Conversion of Alcohols into Esters and Dihydrogen Catalyzed by New Ruthenium Complexes. J. Am. Chem. Soc 2005, 127, 10840–10841. - PubMed
- Owston NA; Parker AJ; Williams JMJ Oxidation of Primary Alcohols to Methyl Esters by Hydrogen Transfer. Chem. Comm 2008, 624–625. - PubMed
- Owston NA; Nixon TD; Parker AJ; Whittlesey MK; Williams JMJ Conversion of Primary Alcohols and Aldehydes into Methyl Esters by Ruthenium-Catalyzed Hydrogen Transfer Reactions. Owston NA; Nixon TD; Parker AJ; Whittlesey MK; Williams JMJ Synthesis 2009, 1578–1581.
- Sølvhø A; Madsen R Dehydrogenative Coupling of Primary Alcohols to Form Esters Catalyzed by a Ruthenium N-Heterocyclic Carbene Complex. Organometallics 2011, 30, 6044–6048.
- Zhao L; He X; Cui T; Nie X; Xu J; Zheng X; Jiang W; Yuan M; Chen H; Fu H; Li R Acceptorless Dehydrogenative Cross-Coupling of Primary Alcohols Catalyzed by an N-Heterocyclic Carbene-Nitrogen-Phosphine Chelated Ruthenium(II) Complex. J. Org. Chem 2022, 87, 4550–4559. - PubMed
-
-
-
For selected examples of iridium-catalyzed oxidative esterification of primary alcohols, see:
- Suzuki T; Matsuo T; Watanabe K; Katoh T Iridium-Catalyzed Oxidative Dimerization of Primary Alcohols to Esters Using 2-Butanone as an Oxidant. Synlett 2005, 1453–1455.
- Izumi A; Obora Y; Sakaguchi S; Ishii Y Oxidative Dimerization of Primary Alcohols to Esters Catalyzed by Iridium Complexes. Tetrahedron Lett. 2006, 47, 9199–9201.
- Arita S; Koike T; Kayaki Y; Ikariya T Aerobic Oxidation of Alcohols with Bifunctional Transition-Metal Catalysts Bearing C–N Chelate Ligands. Chem. Asian J 2008, 3, 1479–1485. - PubMed
- Yamamoto N; Obora Y; Ishii Y The Direct Conversion of Ethanol to Ethyl and Methyl Acetates Catalyzed by Iridium Complex. Chem. Lett 2009, 38, 1106–1107.
- Zweifel T; Naubron J-V; Gruetzmacher H Catalyzed Dehydrogenative Coupling of Primary Alcohols with Water, Methanol, or Amines. Angew. Chem. Int. Ed 2009, 48, 559–563. - PubMed
- Yamamoto N; Obora Y; Ishii Y Iridium-Catalyzed Oxidative Methyl Esterification of Primary Alcohols and Diols with Methanol. J. Org. Chem 2011, 76, 2937–2941. - PubMed
- Onoda M; Fujita K.-i. Dehydrogenative Esterification and Dehydrative Etherification by Coupling of Primary Alcohols Based on Catalytic Function Switching of an Iridium Complex. ChemistrySelect 2022, 7, e202201135.
-
-
-
For selected examples of the ruthenium-catalyzed oxidative lactonization of diols, see:
- Murahashi S-I; Ito K.-i.; Naota T; Maeda Y Tetrahedron Lett. 1981, 22, 5327–5330.
- Shvo Y; Blum Y; Reshef D; Menzin M Catalytic Oxidative Coupling of Diols by Ru3(CO)12. J. Organomet. Chem 1982, 226, C21–C24.
- Ishii Y; Osakada K; Ikariya T; Saburi M; Yoshikawa S Catalytic Enantiotopos Differentiating Dehydrogenation of Prochiral Diols Using Ruthenium Complex with DIOP. Chem. Lett 1982, 11, 1179–1182.
- Ishii Y; Osakada K; Ikariya T; Saburi M; Yoshikawa S Catalytic Regioselective Dehydrogenation of Unsymmetrical α,ω-Diols Using Ruthenium Complexes. Tetrahedron Lett. 1983, 24, 2677–2680.
- Ishii Y; Osakada K; Ikariya T; Saburi M; Yoshikawa S Ruthenium Complex Catalyzed Regioselective Dehydrogenation of Unsymmetrical α,ω-Diols. J. Org. Chem 1986, 51, 2034–2039.
- Murahashi S-I; Naota T; Ito K; Maeda Y: Taki H J. Org. Chem 1987, 52, 4319–4327.
- Isaac I; Aizel G; Stasik I; Wadouachi A; Beaupère D A New Efficient Access to Glycono-1,4-lactones by Oxidation of Unprotected Itols by Catalytic Hydrogen Transfer with RhH(PPh3)4-Benzalacetone System. Synlett 1998, 1998, 475–476.
- Shimizu H; Onitsuka S; Egami H; Katsuki T Ruthenium(salen)-Catalyzed Aerobic Oxidative Desymmetrization of meso-Diols and Its Kinetics. J. Am. Chem. Soc 2005, 127, 5396–5413. - PubMed
- Zhao J; Hartwig JF Acceptorless, Neat, Ruthenium-Catalyzed Dehydrogenative Cyclization of Diols to Lactones. Organometallics 2005, 24, 2441–2446.
- Ito M; Osaku A; Shiibashi A Ikariya, T. An Efficient Oxidative Lactonization of 1,4-Diols Catalyzed by Cp*Ru(PN) Complexes. Org. Lett 2007, 9, 1821–1824. - PubMed
- Tseng K-NT; Kampf JW; Szymczak NK Base-Free, Acceptorless, and Chemoselective Alcohol Dehydrogenation Catalyzed by an Amide-Derived NNN-Ruthenium(II) Hydride Complex. Organometallics 2013, 32, 2046–2049.
- Bhatia A; Kannan M; Muthaiah S Ruthenium-Promoted Acceptorless and Oxidant-Free Lactone Synthesis in Aqueous Medium. Synlett 2019, 30, 721–725.
-
-
-
For selected examples of the iridium-catalyzed oxidative lactonization of diols, see:
- Lin Y; Zhu X; Zhou Y A Convenient Lactonization of Diols to γ- and δ-Lactones Catalysed by Transition Metal Polyhydrides. J. Organomet. Chem 1992, 429, 269–274.
- Suzuki T; Morita K; Tsuchida M; Hiroi K Mild and Chemoselective Synthesis of Lactones from Diols Using a Novel Metal−Ligand Bifunctional Catalyst. Org. Lett 2002, 4, 2361–2363. - PubMed
- Suzuki T; Morita K; Matsuo Y; Hiroi K Catalytic Asymmetric Oxidative Lactonizations of meso-Diols Using a Chiral Iridium Complex. Tetrahedron Lett. 2003, 44, 2003–2006.
- Kosaka M; Sekiguchi S; Naito J; Uemura M; Kuwahara S;Watanabe M; Harada N; Hiroi K Synthesis of Enantiopure Phthalides Including 3-Butylphthalide, a Fragrance Component of Celery Oil, and Determination of Their Absolute Configurations. Chirality 2005, 17, 218–232. - PubMed
- Fujita K.-i.; Ito W; Yamaguchi R Dehydrogenative Lactonization of Diols in Aqueous Media Catalyzed by a Water-Soluble Iridium Complex Bearing a Functional Bipyridine Ligand. ChemCatChem 2014, 6, 109–112.
- Moritani J; Hasegawa Y; Kayaki Y; Ikariya T Aerobic Oxidative Desymmetrization of meso-Diols with Bifunctional Amidoiridium Catalysts Bearing Chiral N-Sulfonyldiamine Ligands. Tetrahedron Lett. 2014, 55, 1188–1191.
-
-
-
For general reviews on the dehydrogenative conversion of primary alcohols and diols to esters and lactones, see:
- Naota T; Takaya H; Murahashi S-I Ruthenium-Catalyzed Reactions for Organic Synthesis. Chem. Rev 1998, 98, 2599–2660. - PubMed
- Dobereiner GE; Crabtree RH Dehydrogenation as a Substrate-Activating Strategy in Homogeneous Transition-Metal Catalysis. Chem. Rev 2010, 110, 681–703. - PubMed
- Suzuki T Organic Synthesis Involving Iridium-Catalyzed Oxidation. Chem. Rev 2011, 111, 1825–1845. - PubMed
- Luo F; Pan C; Cheng J Recent Advances in Transition-Metal-Catalyzed Esterification. Synlett 2012, 23, 357–366.
- Tang S; Yuan J; Liu C; Lei A Direct Oxidative Esterification of Alcohols. Dalton Trans. 2014, 43, 13460–13470. - PubMed
-
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

