Impact of point-of-care maternal viral load testing at delivery on vertical HIV transmission risk assessment and neonatal prophylaxis: a cluster randomized trial
- PMID: 40719344
- PMCID: PMC12302277
- DOI: 10.1002/jia2.70021
Impact of point-of-care maternal viral load testing at delivery on vertical HIV transmission risk assessment and neonatal prophylaxis: a cluster randomized trial
Abstract
Introduction: Despite global reductions in vertical HIV transmission (VHT), 120,000 children newly acquired HIV in 2023. High maternal viral load (VL) is a major risk factor for VHT. We estimated the impact of point-of-care (PoC) maternal VL testing at delivery in profiling the risk of VHT and its impact on appropriate postnatal prophylaxis for infants born to women living with HIV (WLWH).
Methods: The cluster-randomized LIFE (Long term Impact on inFant hEalth) study was conducted at 28 health facilities in Tanzania and Mozambique from 2019 to 2021. At delivery, the intervention arm applied PoC maternal VL plus clinical criteria for VHT risk assessment, while the control arm used clinical criteria only. In Tanzania, both arms provided ePNP based on maternal risk factors, while Mozambique provided ePNP universally. We used mixed effects logistic regression to estimate the intervention effect on the proportion of infants at high risk (Tanzania and Mozambique) and infants at high risk receiving ePNP (Tanzania only).
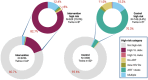
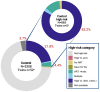
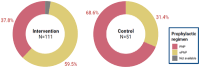
Results: A total of 6467 WLWH were enrolled: 66.3% were diagnosed before the third trimester, 99% were on antiretroviral therapy and 78% were virally suppressed at delivery. Of 6564 newborns of WLWH included, 774 (11.7%) were identified to be at a high risk: 629 (19.3%) versus 145 (4.4%) in intervention and control arms, respectively; p<0.0001. In the intervention arm, 520 (82.7%) infants at high risk were classified only based on maternal PoC VL at delivery. In the control arm, 720 (21.8%) additional infants at high risk would have been identified if their mothers had received PoC VL assessment. In Tanzania, infants at high risk in the intervention arm were significantly more likely to receive ePNP: 59.5% versus 31.4% (OR 4.42, 95% CI: 1.09, 17.89). However, 40.5% from intervention arm and 68.6% from control arm did not receive ePNP despite high-risk classification at delivery.
Conclusions: PoC maternal VL testing at delivery significantly increased the proportion of infants identified to be at high risk. Infants at high risk whose mothers received PoC VL at delivery were more often initiated on ePNP. However, the linkage of infants at high risk to appropriate prophylaxis remains suboptimal, warranting consideration of universal ePNP.
Keywords: HIV acquisitions; infant; newborn; point‐of‐care systems; risk assessment; viral load.
© 2025 The Author(s). Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Conflict of interest statement
We declare that none of the authors have any competing interests that have influenced the conduct of the study and write‐up of this manuscript.
Figures
References
-
- UNAIDS . The urgency of now: AIDS at a crossroads. Jt United Nations Program HIV/AIDS. 2024;150. Available from: https://www.unaids.org/sites/default/files/media_asset/2024‐unaids‐globa...
-
- da Saúde M. Relatório Anual 2022. 2023.
-
- UNICEF . The mother of all prevention. UNICEF United Republic of Tanzania [Internet]. [cited 2022 Sep 21]. Available from: https://www.unicef.org/tanzania/stories/mother‐all‐prevention
-
- PMTCT annual report of Tanzania. 2023.
-
- Bardeskar NS, Ahir‐Bist SP, Mehta PR, Samant‐Mavani P, Nanavati R, Mania‐Pramanik J. Anti‐retroviral therapy failure in HIV‐1 infected pregnant women and its associated risk of HIV transmission. Arch Gynecol Obstet. 2020;302(5):1229–1235. - PubMed

