Systematic pan-cancer analysis identified NCOA4 as an immunological and prognostic biomarker and validated in lung adenocarcinoma
- PMID: 40719993
- PMCID: PMC12304365
- DOI: 10.1007/s12672-025-03064-3
Systematic pan-cancer analysis identified NCOA4 as an immunological and prognostic biomarker and validated in lung adenocarcinoma
Abstract
Background: Cancer immunotherapy has revolutionized treatment, yet predicting patient responses remains challenging. The potential of nuclear receptor coactivator 4 (NCOA4) as a biomarker has been identified in different types of cancer. However, its role as an immunological and prognostic factor across cancers, particularly lung adenocarcinoma (LUAD), still needs to be fully understood. This study aims to address this gap by systematically analyzing NCOA4's expression and its correlation with clinical outcomes and immune responses in pan-cancer.
Objective: To evaluate NCOA4 as a prognostic biomarker and explore its association with immune microenvironments across different cancer types, specifically focusing on LUAD.
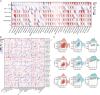
Methods: We comprehensively analyzed data from the CCLE, GTEx, and TCGA databases. NCOA4 expression was analyzed in both normal and tumor tissues, and its correlation with overall survival was assessed using univariate Cox regression and Kaplan-Meier analysis. Immune infiltration was evaluated using the ESTIMATE, TIMER, and xCELL algorithms. We comprehensively analyzed its correlation with six genomic instability markers and the DNA methylation- and mRNA-based stemness index in certain tumors to investigate the potential role of NCOA4 in mediating cancer genomic heterogeneity and stemness. PPI network analysis and KEGG/GO enrichment analysis were conducted to identify associated proteins and pathways. LUAD samples were stained using immunohistochemistry (IHC). TIDE algorithm was used to predict immune checkpoint blockade (ICB) response within the TCGA-LUAD cohort.
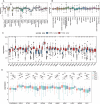
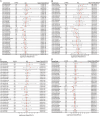
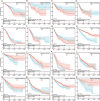
Results: NCOA4 expression was significantly higher in tumor tissues than normal tissues in 18 cancer types, including LUAD, while it was lower in 5. Survival outcomes in specific cancers were found to be inversely associated with NCOA4 expression, as indicated by the results of univariate Cox regression and Kaplan-Meier analysis. Analysis of immune infiltration demonstrated a significant association between NCOA4 and the presence of immune cells, including CD8 + T cells, neutrophils, and dendritic cells. Genomic instability markers, including TMB and MSI, showed significant correlations with NCOA4 expression, indicating a potential role in immunotherapy response. Stemness index analyses suggested NCOA4's involvement in regulating tumor stemness. PPI network and KEGG/GO enrichment analyses implicated NCOA4 in immune-related biological processes and pathways. IHC staining of LUAD tissues confirmed higher NCOA4 expression in tumors versus non-cancerous tissues. The TIDE algorithm predicted that higher NCOA4 expression levels, as indicated by elevated TIDE scores, were associated with poorer ICB response rates in the TCGA-LUAD cohort.
Conclusion: NCOA4 exhibits context-dependent associations with prognosis, immune infiltration, and genomic instability across cancers. While experimental validation in LUAD supports its candidacy as a biomarker, mechanistic studies are required to establish causal relationships. These findings highlight NCOA4's potential utility in stratifying patients for immunotherapy and ferroptosis-targeted therapies.
Keywords: Immunological biomarker; Immunotherapy; Lung adenocarcinoma; NCOA4; Prognostic biomarker; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures involving human tissues were performed in accordance with the ethical standards of the Institutional Review Board of Tangdu Hospital (No. 202003-019) and the 1964 Helsinki Declaration. Informed consent was waived due to the retrospective nature of the study. Informed consent was waived by the Institutional Review Board due to the retrospective design and anonymized data usage. Consent for publication: All the listed authors have carefully reviewed and approved this manuscript. We all agree to submit it to Discover Oncology for publication and are aware of and accept the journal’s publication policies. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Interplay between tumor mutation burden and the tumor microenvironment predicts the prognosis of pan-cancer anti-PD-1/PD-L1 therapy.Front Immunol. 2025 Jul 24;16:1557461. doi: 10.3389/fimmu.2025.1557461. eCollection 2025. Front Immunol. 2025. PMID: 40777041 Free PMC article.
-
Comprehensive pan-cancer analysis reveals NTN1 as an immune infiltrate risk factor and its potential prognostic value in SKCM.Sci Rep. 2025 Jan 25;15(1):3223. doi: 10.1038/s41598-025-85444-x. Sci Rep. 2025. PMID: 39863609 Free PMC article.
-
Integrated pan-cancer analysis of ADM's role in prognosis, immune modulation and resistance.Front Immunol. 2025 Jun 3;16:1573250. doi: 10.3389/fimmu.2025.1573250. eCollection 2025. Front Immunol. 2025. PMID: 40529377 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. 10.3322/caac.21834. - PubMed
-
- Lei G, Zhuang L, Gan B. The roles of ferroptosis in cancer: tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell. 2024;42(4):513–34. 10.1016/j.ccell.2024.03.011. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
