Comparative Evaluation of Lipopolysaccharide Administration Methods to Induce Acute Lung Injury in Murine Models: Efficacy, Consistency, and Technical Considerations
- PMID: 40720189
- PMCID: PMC12306704
- DOI: 10.1097/CCE.0000000000001292
Comparative Evaluation of Lipopolysaccharide Administration Methods to Induce Acute Lung Injury in Murine Models: Efficacy, Consistency, and Technical Considerations
Abstract
Context: Direct preclinical lipopolysaccharide acute lung injury (ALI) models are commonly used to study acute respiratory distress syndrome. Differences in lipopolysaccharide delivery methods may impact lung injury severity and reproducibility.
Hypothesis: We hypothesized that the severity and variability of ALI outcomes in mice would differ depending on the technique of lipopolysaccharide administration.
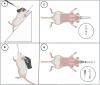
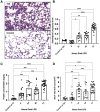
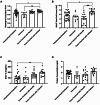
Methods and models: Male and female C57BL/6 mice were administered lipopolysaccharide (2.25 mg/kg) via four methods: 1) intratracheal intubation; 2) intranasal; 3) surgical transtracheal by either needle puncture; or 4) by catheter. ALI severity and variability were assessed at 72 hours post-lipopolysaccharide via histological scoring and bronchoalveolar lavage fluid (BALF) analysis (total protein, cell counts, interleukin-6 [IL-6]). The relative distribution of Evans Blue dye was also assessed for each model (lungs vs. stomach).
Results: Distinct lung injury patterns were observed between the four methods. The transtracheal with catheter method demonstrated significantly greater lung injury scores than the intratracheal intubation and intranasal techniques. Both transtracheal methods produced greater alveolar neutrophil counts, increased proteinaceous debris, fewer hyaline membranes, and lower variability than non-surgical techniques. The transtracheal with catheter method produced higher BALF total cell counts and IL-6 levels than intratracheal intubation. Transtracheal methods also resulted in more localized Evans Blue dye distribution in the lungs. Male mice exhibited more severe lung injury scores and higher BALF protein concentrations than females.
Interpretation and conclusions: This study demonstrates that the choice of technique to administer lipopolysaccharide impacts injury severity, phenotype, and variability. The surgical transtracheal with catheter technique produced the most robust and least variable ALI phenotype; however, this technique is associated with increased procedural complexity. Our results will allow researchers to tailor their model choice to align with their specific study objectives.
Keywords: acute lung injury; acute respiratory distress syndrome; biological sex; disease models; lipopolysaccharide; sex factors.
Copyright © 2025 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
Ms. Kuhar was supported by a Canada Graduate Scholarship with the Canadian Institutes of Health Research (CIHR). Dr. Lalu wassupported by the Ottawa Hospital Anesthesia Alternate Funds Association, the Canadian Anesthesiologists’ Society Career Investigator Award, and a University of Ottawa Junior Research Chair in Innovative Translational Research. Dr. Zhang holds a Robert and Dorothy Pitts Chair in Acute Care and Emergency Medicine, a joint Hospital-University Endowed Chair between the University of Toronto, Unity Health Toronto, and the St. Michael’s Hospital Foundation. Mr. Jeffers is supported by the CIHR and Vanier Canada Graduate Scholarship. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures

References
-
- Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, et al. : Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353:1685–1693 - PubMed
-
- Mizgerd JP, Skerrett SJ: Animal models of human pneumonia. Am J Physiol Lung Cell Mol Physiol 2008; 294:L387–L398 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

