Phosphoglycerate mutase regulates Treg differentiation through control of serine synthesis and one-carbon metabolism
- PMID: 40720256
- PMCID: PMC12303568
- DOI: 10.7554/eLife.104423
Phosphoglycerate mutase regulates Treg differentiation through control of serine synthesis and one-carbon metabolism
Abstract
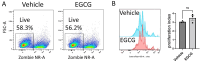
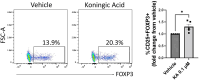
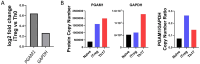
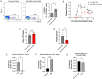
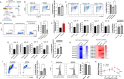
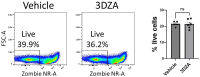
The differentiation and suppressive functions of regulatory CD4 T cells (Tregs) are supported by a broad array of metabolic changes, providing potential therapeutic targets for immune modulation. In this study, we focused on the regulatory role of glycolytic enzymes in Tregs and identified phosphoglycerate mutase (PGAM) as being differentially overexpressed in Tregs and associated with a highly suppressive phenotype. Pharmacologic or genetic inhibition of PGAM reduced Treg differentiation and suppressive function while reciprocally inducing markers of a pro-inflammatory, T helper 17 (Th17)-like state. The regulatory role of PGAM was dependent on the contribution of 3-phosphoglycerate (3 PG), the PGAM substrate, to de novo serine synthesis. Blocking de novo serine synthesis from 3 PG reversed the effect of PGAM inhibition on Treg polarization, while exogenous serine directly inhibited Treg polarization. Additionally, altering serine levels in vivo with a serine/glycine-free diet increased peripheral Tregs and attenuated autoimmunity in a murine model of multiple sclerosis. Mechanistically, we found that serine limits Treg polarization by contributing to one-carbon metabolism and methylation of Treg-associated genes. Inhibiting one-carbon metabolism increased Treg polarization and suppressive function both in vitro and in vivo in a murine model of autoimmune colitis. Our study identifies a novel physiologic role for PGAM and highlights the metabolic interconnectivity between glycolysis, serine synthesis, one-carbon metabolism, and epigenetic regulation of Treg differentiation and suppressive function.
Keywords: PGAM; T cell biology; Treg; glycolysis; immunology; immunometabolism; inflammation; mouse; phosphoglycerate mutase; serine synthesis.
© 2025, Godfrey et al.
Conflict of interest statement
WG, JL, SS, KC, XD, CA, VP, AM, PK, NP No competing interests declared, MK Has received ad hoc consulting fees from Biogen Idec, Genentech, Janssen Pharmaceuticals, Novartis, OptumRx, and TG Therapeutics on topics unrelated to this manuscript
Figures




Update of
-
Phosphoglycerate mutase regulates Treg differentiation through control of serine synthesis and one-carbon metabolism.bioRxiv [Preprint]. 2025 Jun 10:2024.06.23.600101. doi: 10.1101/2024.06.23.600101. bioRxiv. 2025. Update in: Elife. 2025 Jul 28;14:RP104423. doi: 10.7554/eLife.104423. PMID: 38979375 Free PMC article. Updated. Preprint.
References
-
- Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, Wang Z, Kopinski PK, Quinn WJ, Wang L, Akimova T, Liu Y, Bhatti TR, Han R, Laskin BL, Baur JA, Blair IA, Wallace DC, Hancock WW, Beier UH. Foxp3 Reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metabolism. 2017;25:1282–1293. doi: 10.1016/j.cmet.2016.12.018. - DOI - PMC - PubMed
-
- Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bähre H, Tschirner SK, Gorinski N, Gohmert M, Mayer CT, Huehn J, Ponimaskin E, Abraham WR, Müller R, Lochner M, Sparwasser T. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nature Medicine. 2014;20:1327–1333. doi: 10.1038/nm.3704. - DOI - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

