Structural Properties and Stability of Proteins in Dihydrolevoglucosenone/Water Mixtures
- PMID: 40720355
- PMCID: PMC12337083
- DOI: 10.1021/acs.jpcb.5c02271
Structural Properties and Stability of Proteins in Dihydrolevoglucosenone/Water Mixtures
Abstract
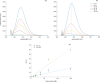
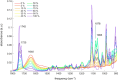
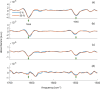
Dihydrolevoglucosenone (DHL) shows great promise as an alternative to conventional toxic organic solvents widely used for industrial purposes. In this framework, evaluating the potential of DHL (commercially known as Cyrene) as a solvent for dissolving proteins is of great importance. Here, the effect of DHL/water mixtures on protein stability and solubility has been assessed. Several proteins, namely, hemoglobin, ferritin, ribonuclease, and albumin, were readily dissolved in buffer solutions containing up to 50-60% DHL and were stable at room temperature, as indicated by gel electrophoresis and matrix-assisted laser desorption/ionization analysis. Turbidimetry assays were performed in order to assess the solubility limitations derived from DHL/water mixtures. Finally, protein secondary structures in such mixtures, investigated by attenuated total reflectance Fourier-transform infrared spectroscopy, were found to be comparable to those obtained in phosphate buffer up to 50% DHL/water, with small spectral changes in the case of ribonuclease. DHL/water mixtures may thus represent highly convenient solvents for studies of protein chemistry.
Figures



References
-
- Court, G. R. ; Howard Lawrence, C. ; Raverty, W. D. ; Duncan, A. J. . Method for Converting Lignocellulosic Materials into Useful Chemicals. US20120111714A1, 2012.
-
- Brouwer T., Schuur B.. Dihydrolevoglucosenone (Cyrene), a Biobased Solvent for Liquid–Liquid Extraction Applications. ACS Sustain Chem. Eng. 2020;8(39):14807–14817. doi: 10.1021/acssuschemeng.0c04159. - DOI
-
- Duval A., Avérous L.. Dihydrolevoglucosenone (Cyrene) as a Versatile Biobased Solvent for Lignin Fractionation, Processing, and Chemistry. Green Chem. 2022;24(1):338–349. doi: 10.1039/D1GC03395F. - DOI
-
- Meng X., Pu Y., Li M., Ragauskas A. J.. A Biomass Pretreatment Using Cellulose-Derived Solvent Cyrene. Green Chem. 2020;22(9):2862–2872. doi: 10.1039/D0GC00661K. - DOI
-
- Sullivan C., Zhang Y., Xu G., Christianson L., Luengo F., Halkoski T., Gao P.. Cyrene Blends: A Greener Solvent System for Organic Syntheses. Green Chem. 2022;24(18):7184–7193. doi: 10.1039/D2GC01911F. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

