The plasmepsin-piperaquine paradox persists in Plasmodium falciparum
- PMID: 40720544
- PMCID: PMC12321132
- DOI: 10.1371/journal.ppat.1012779
The plasmepsin-piperaquine paradox persists in Plasmodium falciparum
Abstract
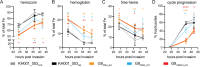
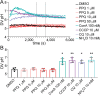
Malaria remains a pressing global health challenge, with rising drug resistance threatening current treatment strategies. Partial resistance to dihydroartemisinin-piperaquine (DHA-PPQ) has emerged in Southeast Asia, particularly in Plasmodium falciparum strains from Cambodia. While artemisinin partial resistance is associated with mutations in kelch13, reduced PPQ sensitivity has been linked to increased copy numbers of the aspartic protease genes plasmepsin II and III and mutations in the chloroquine resistance transporter. In this study, we demonstrate the effective use of CRISPR-Cas9 technology to generate single knockouts (KO) of plasmepsin II and plasmepsin III, as well as a double KO of both genes, in two isogenic Cambodian parasites with varying numbers of plasmepsin gene copies. The deletion of plasmepsin II and/or III increased parasite sensitivity to PPQ. We explored several hypotheses to understand how an increased plasmepsin gene copy number might influence parasite survival under high PPQ pressure. Our findings indicate that protease inhibitors have a minimal impact on parasite susceptibility to PPQ. Additionally, parasites with higher plasmepsin gene copy numbers did not exhibit significantly increased hemoglobin digestion, differences in peptide composition, nor did they produce different amounts of free heme following PPQ treatment compared to wildtype (single copy) parasites. Interestingly, hemoglobin digestion was slowed in parasites with plasmepsin II deletions. We also found that culturing parasites with different plasmepsin II and III copies in amino acid-limited media had little impact on parasite sensitivity to high-dose PPQ. By treating parasites with modulators of digestive vacuole (DV) homeostasis, we found that changes in DV pH potentially affect their response to PPQ. Our research highlights the crucial role of increased plasmepsin II and III gene copy numbers in modulating response to PPQ and begins to uncover the molecular and physiological mechanisms underlying the contribution of plasmepsin II and III amplification to PPQ resistance in Cambodian parasites.
Copyright: © 2025 Walsh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Organization WH. World malaria report 2023. 2023.
-
- van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, et al. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis. 2019. Epub 2019 Jul 28. 10.1016/s1473-3099(19)30391-3 - DOI - PMC - PubMed
-
- Leang R, Barrette A, Bouth DM, Menard D, Abdur R, Duong S, et al. Efficacy of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob Agents Chemother. 2013;57(2):818–26. doi: 10.1128/AAC.00686-12 - DOI - PMC - PubMed
-
- Leang R, Taylor WRJ, Bouth DM, Song L, Tarning J, Char MC, et al. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in Western Cambodia: Dihydroartemisinin-piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59(8):4719–26. doi: 10.1128/AAC.00835-15 - DOI - PMC - PubMed
-
- Duru V, Khim N, Leang R, Kim S, Domergue A, Kloeung N, et al. Plasmodium falciparum dihydroartemisinin-piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: retrospective and prospective investigations. BMC Med. 2015;13:305. doi: 10.1186/s12916-015-0539-5 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

