Targeting translation initiation yields fast-killing therapeutics against the zoonotic parasite Cryptosporidium parvum
- PMID: 40720562
- PMCID: PMC12313074
- DOI: 10.1371/journal.ppat.1012881
Targeting translation initiation yields fast-killing therapeutics against the zoonotic parasite Cryptosporidium parvum
Abstract
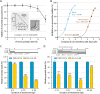
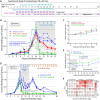
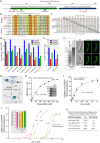
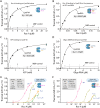
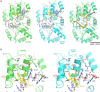
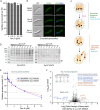
Cryptosporidium parvum is a zoonotic apicomplexan that causes moderate-to-severe watery diarrhea in children, immunocompromised patients, and neonatal ruminants, yet no fully effective drug is available. We show that the parasite's eukaryotic initiation factor 4A (CpeIF4A; a DEAD-box RNA helicase in the eIF4F translation-initiation complex) can be exploited as a fast-killing therapeutic target. Rocaglamide A (Roc-A), a plant-derived rocaglate, binds the CpeIF4A-RNA-ATP complex with high affinity (Kd = 33.7 nM) and blocks protein synthesis in excysting sporozoites (IC50 ≈ 3.7 µM). In host-cell culture, Roc-A suppresses intracellular growth with nanomolar potency (EC50 = 1.77 nM) and a selectivity index exceeding 56,000 in HCT-8 cells and 1,400 in HepG2 cells. A five-day oral regimen (0.5 mg/kg/d) reduced oocyst shedding by >90% within 48 h in interferon-γ-knockout mice and by 70-90% from day 2 onward without rebound during a 15-day follow-up in NCG mice. Two amino-acid differences at the Roc-A binding surface (D165 and V192 in CpeIF4A vs. N167 and D194 in the human ortholog) offer a foothold for medicinal optimization toward greater parasite selectivity. These findings establish translation initiation as an unexplored but tractable pathway for anti-cryptosporidial drug discovery and position Roc-A as a promising lead compound.
Copyright: © 2025 Li et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

