SARS-CoV-2 uptake and inflammatory response in senescent endothelial cells are regulated by the BSG/VEGFR2 pathway
- PMID: 40720650
- PMCID: PMC12337311
- DOI: 10.1073/pnas.2502724122
SARS-CoV-2 uptake and inflammatory response in senescent endothelial cells are regulated by the BSG/VEGFR2 pathway
Abstract
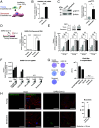
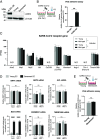
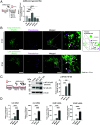
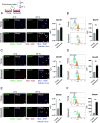
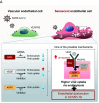
Aging is a risk factor for severe COVID-19, characterized by vascular endothelial dysfunction. Although possible susceptibility of vascular endothelial cells (ECs) to SARS-CoV-2 infection has been suggested, the details of entry into cells have not been clarified. Previously, we reported that in an aged mouse model of severe COVID-19, ECs show a massive viral uptake and inflammatory response. Here, we focused on the endocytic capacity of senescent ECs. We found that the senescent ECs showed high endocytic capacity and SARS-CoV-2 virus uptake. This triggers an nuclear factor-kappa B (NF-κB) pathway-mediated inflammatory response. Further, Basigin enhanced endocytosis in the senescent ECs by activating the intracellular vascular endothelial growth factor signaling. Thus, EC senescence is associated with enhanced SARS-CoV-2 endocytosis and subsequent vascular endothelial dysfunction. This could prove a potential target for treating severe COVID-19 in older adults.
Keywords: BSG; COVID-19; SARS-CoV-2; senescence; vascular endothelial cell.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- WHO, World Health Organization 2023 data.who.int, WHO Coronavirus (COVID-19) dashboard. https://data.who.int/dashboards/covid19/. Accessed 24 October 2024.
MeSH terms
Substances
Grants and funding
- JP20fk0108537h0001/Japan Agency for Medical Research and Development (AMED)
- JP22wm0325053h0001/Japan Agency for Medical Research and Development (AMED)
- JP243fa627005/Japan Agency for Medical Research and Development (AMED)
- JP23wm0125008/Japan Agency for Medical Research and Development (AMED)
- JP22K19610/OU | Research Institute for Microbial Diseases, Osaka University (RIMD)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

