Proposed Research Criteria for Mild Motor Impairment as a Prodromal Syndrome in Amyotrophic Lateral Sclerosis
- PMID: 40720712
- PMCID: PMC12296636
- DOI: 10.1212/WNL.0000000000213917
Proposed Research Criteria for Mild Motor Impairment as a Prodromal Syndrome in Amyotrophic Lateral Sclerosis
Abstract
Background and objectives: The presymptomatic stage of amyotrophic lateral sclerosis (ALS) is typically assumed to be clinically silent. Our previous work, however, has revealed evidence of a prodromal stage, termed mild motor impairment (MMI). In this study, we propose operational criteria for MMI, describe its frequency in a genetically diverse cohort, and examine the association between MMI and time to ALS phenoconversion.
Methods: Pre-Symptomatic Familial ALS (Pre-fALS) study, initiated in 2007, is a longitudinal natural history and biomarker study of unaffected carriers of any ALS-associated pathogenic variant. Proposed research criteria for MMI were developed based on clinical review of case histories of ALS phenoconverters in the Pre-fALS study. These criteria were then systematically applied to the remaining Pre-fALS cohort. Among phenoconverters and presymptomatic carriers, cumulative probabilities of phenoconversion were estimated for those with and without MMI at baseline. Cox proportional hazard models evaluated associations between risk factors (MMI, age, genotype) and time from baseline to ALS phenoconversion.
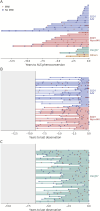
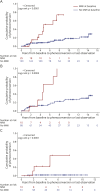
Results: The study cohort includes 49 controls (mean age 38.9, 61% female), 153 presymptomatic carriers (mean age 43.1, 61% female), and 31 ALS phenoconverters (mean age 54.4, 58% female). Seven criteria for MMI are proposed, each reflecting a different pattern of upper/lower motor neuron signs, without corresponding weakness, in 1 or more of 4 topographic regions. MMI, defined as meeting ≥1 of these criteria, was observed at 1 or more study visits in 17 of 31 phenoconverters (55%) before clinically manifest ALS, 66 of 153 presymptomatic carriers (43%) who had not yet phenoconverted, and 7 of 49 controls (14%). Among all mutation carriers, the 25th percentile (95% CI) of time to phenoconversion was, respectively, 3.7 (1.6-4.7) and 14.1 (8.9-∞) years for those with and without MMI at baseline, with a hazard ratio of 5.1 (95% CI 2.2-12.2, p < 0.0001). Moreover, the hazard rate of phenoconversion was 1.7 times higher for every 10-year increase in age and varied by genotype.
Discussion: MMI is a recognizable syndrome. Although nonspecific, it identifies presymptomatic carriers at elevated risk of ALS phenoconversion. Combining MMI with emerging biomarkers of underlying pathology may help differentiate those who are prodromal from those who are not prodromal for ALS. Characterization and study of MMI, including replication in other studies, may empower early therapeutic intervention and ALS prevention efforts.
Conflict of interest statement
M. Benatar has served on advisory boards for Alaunos, Alector, Alexion, Amgen, Annexon, Arrowhead, Biogen, Bristol Myers Squibb, Canopy, Cartesian, CorEvitas, Denali, Eli Lilly, Immunovant, Janssen, Merck, Novartis, Prilenia, Roche, Sanofi, Takeda, UCB, uniQure, and Woolsey. The University of Miami has licensed intellectual property to Biogen to support design of the ATLAS study. V. Granit is currently employed by, and has stock holdings at, Biohaven Pharmaceuticals. P.M. Andersen has served on advisory boards for Biogen, Avrion, Arrowhead, Regeneron, uniQure, Orphazyme, and Roche. All other authors report no disclosures relevant to this manuscript. Go to
Figures
Comment in
- Neurology. 105:e214058.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
