Excessive glycosylation drives thoracic aortic aneurysm formation through integrated stress response
- PMID: 40720766
- PMCID: PMC12665370
- DOI: 10.1093/eurheartj/ehaf556
Excessive glycosylation drives thoracic aortic aneurysm formation through integrated stress response
Abstract
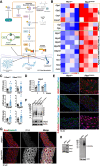
Background and aims: Thoracic aortic aneurysms and dissections (TAADs) are depicted by aortic medial degeneration characterized by glycan-rich matrix accumulation. Marfan syndrome (MFS) is the most common inherited connective tissue disorder associated with TAAD. Although vascular smooth muscle cell metabolic dysfunction has emerged as a pathogenic driver of TAAD, surgical repair remains the mainstay of treatment. This study aimed to investigate the role of the hexosamine biosynthetic pathway (HBP) in sporadic and genetic TAAD pathophysiology.
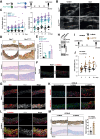
Methods: Hexosamine biosynthetic pathway activation was analysed in aortas from an MFS mouse model, a β-aminopropionitrile-induced non-genetic TAAD model, and patients with sporadic TAAD using transcriptomic and metabolomic approaches. Aortic dilatation was monitored by ultrasound imaging. Pharmacological inhibition of HBP and integrated stress response (ISR) was performed to assess their therapeutic potential.
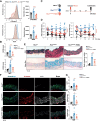
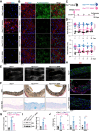
Results: Hexosamine biosynthetic pathway was up-regulated in both an MFS mouse model and β-aminopropionitrile-induced TAAD, as well as in aortic samples from MFS and sporadic TAAD patients. Enhanced HBP activity contributed to aortic dilatation and medial degeneration via vascular smooth muscle cell dysfunction and ISR activation. Inhibition of HBP or ISR reversed these effects in the MFS model.
Conclusions: The HBP-ISR axis drives medial degeneration in TAAD. These findings identify HBP and ISR as a potential target in TAAD of both genetic and non-genetic origin.
Keywords: Aortic medial degeneration; Hexosamine Biosynthetic pathway; Integrated stress response; Marfan Syndrome; Thoracic aortic aneurysm.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures




References
MeSH terms
Substances
Grants and funding
- RYC2021-033343-I/Conchita-Rábago Foundation
- MCIN/AEI/10.13039/501100011033/FEDER,UE/Spanish Science Ministry
- Fundación MERCK-FEDER 'Investigación clínica en enfermedades raras 2022'
- Marfan Spanish Association
- Y2020/BIO-6350/Comunidad de Madrid
- PID2022-141169OB-I00/Spanish Ministerio de Ciencia e Innovación
- PI20/01103/Instituto de Salud Carlos III
- PI23/00100/Instituto de Salud Carlos III
- CP22/00100/Instituto de Salud Carlos III
- ISCIII/FEDER PI22/00233/Instituto de Salud Carlos III
- CB16/11/00264/Instituto de Salud Carlos III
- European Regional Development Fund
- European Social Fund
- PID2022-136979OB-I00/Spanish Science Ministry
- Fondo de Investigaciones Sanitarias
- Spanish Society of Cardiology
- PI20/00923/Spanish Health Ministry
- PI21/00084 FIS/Spanish Health Ministry
- INNVAL21/24/Health Research Institute Valdecilla
- PI21/01126/Spanish Health Ministry
- FIS22/00140/Spanish Health Ministry
- HR18-00068/'La Caixa' Banking Foundation
- PID2021-122388OB-100/Spanish Ministerio de Ciencia e Innovación
- 202334-31/Fundació La Marató TV3
- BES-2016-077649/Spanish Ministerio de Ciencia e Innovación contract FPI
- Consejo Superior de Investigaciones Científicas
- Universidad Autónoma de Madrid
- CEX2021-001154-S/CBMSO
- MICIN/AEI/10.13039/501100011033/CBMSO
- ERC-2021-CoG 101044248/ERC_/European Research Council/International
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

