Engagement With Digital Adherence Technologies as Measures of Intervention Fidelity Among Adults With Drug-Susceptible Tuberculosis and Health Care Providers: Descriptive Analysis Using Data From Cluster-Randomized Trials in Five Countries
- PMID: 40720892
- PMCID: PMC12303541
- DOI: 10.2196/62881
Engagement With Digital Adherence Technologies as Measures of Intervention Fidelity Among Adults With Drug-Susceptible Tuberculosis and Health Care Providers: Descriptive Analysis Using Data From Cluster-Randomized Trials in Five Countries
Abstract
Background: Digital adherence technologies (DATs) are promising tools for supporting tuberculosis (TB) treatment. DATs can serve as reminders for people with TB to take their medication and act as proxies for adherence monitoring. Strong engagement with DATs, from both the person with TB and health care provider (HCP) perspectives, is essential for ensuring intervention fidelity. The Adherence Support Coalition to End TB (ASCENT) project evaluated 2 types of DATs, pillboxes and medication labels (99DOTS), in cluster-randomized trials across 5 countries.
Objective: This study aims to investigate participant and HCP engagement with DATs for TB treatment, stratified by DAT type and country.
Methods: This study is a subanalysis of data generated through the ASCENT trials, which enrolled adults with drug-susceptible TB. A digital dose was defined as either a pillbox opening (for pillbox users) or a dosing confirmation SMS text message sent by the participant (for label users), both of which were recorded on the adherence platform. Descriptive analysis was used to provide an overview of dose-day outcomes. DAT engagement was assessed from both participant and HCP perspectives. To enhance participant engagement, we summarized the frequency of digital engagement overall and by treatment phase, as well as the frequency of consecutive days without engagement. For HCP engagement, we summarized the frequency of doses added manually, the number of days between the actual dose day and when a manual dose was added, and instances of consecutive manual dosing lasting more than 3 and more than 7 days, where doses were added more than 1 week after the dose day.
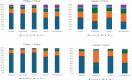
Results: Of the 9511 participants included, 6719 (70.64%) were using the pillbox, 3544 (37.26%) were female, and the median age was 40 years. Across DAT types, there were 1,384,879 dose days, with 973,876 (70.32%) contributed by pillbox users. Of all dose days, 1,165,195 (84.14%) were recorded as digital, 156,664 (11.31%) as manual, 59,045 (4.26%) had no information, and 3975 (0.29%) were confirmed as missed. Digital dosing decreased slightly from the intensive to the continuation phase. The percentage of digital dose days was higher among pillbox users (851,496/973,876, 87.43%) compared with label users (313,699/411,003, 76.33%). Among label users, manual dosing was most common in the Philippines (37,919/171,786, 22.07%) and least common in Tanzania (11,108/76,231, 14.57%). Among pillbox users, manual dosing was most common in the Philippines (24,015/208,130, 11.54%) and Ukraine (13,209/111,901, 11.80%). Overall, 512 out of 2792 (18.34%) label users and 588 out of 6719 (8.75%) pillbox users experienced a run of more than 7 consecutive nondigital dose days that were resolved more than 1 week after the dose day. The highest occurrence was observed in the Philippines (368/1142, 32.22%, for label users and 224/1351, 16.58%, for pillbox users).
Conclusions: There was considerable variation in DAT engagement across countries and DAT types, reflecting differences in how the intervention was implemented. Further refinement of the intervention and improvements in its delivery may be necessary to enhance outcomes.
Keywords: DAT engagement; digital adherence technology; digital dosing; intervention fidelity; manual dosing; tuberculosis.
© Jason Alacapa, Amare Worku Tadesse, Natasha Deyanova, Tanyaradzwa Dube, Andrew Mganga, Rachel Powers, Job van Rest, Norma Madden, Egwuma Efo, Salome Charalambous, Kristian van Kalmthout, Degu Jerene, Katherine Fielding. Originally published in JMIR Public Health and Surveillance (https://publichealth.jmir.org).
Conflict of interest statement
Figures


References
-
- Global tuberculosis report 2024. World Health Organization (WHO) 2024. [08-07-2025]. https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-heal... URL. Accessed.
-
- Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 update. World Health Organization (WHO) 2017. [08-07-2025]. https://iris.who.int/bitstream/handle/10665/255052/9789241550000-eng.pdf URL. Accessed.
-
- Mohamed MS, Zary M, Kafie C, et al. The impact of digital adherence technologies on health outcomes in tuberculosis: a systematic review and meta-analysis. medRxiv. 2024 Feb 3; doi: 10.1101/2024.01.31.24302115. Preprint posted online on. doi. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

