Regulatory B cells drive immune evasion in the tumor microenvironment and are involved peritoneal metastasis in gastric cancer
- PMID: 40721618
- PMCID: PMC12304303
- DOI: 10.1038/s41598-025-11887-x
Regulatory B cells drive immune evasion in the tumor microenvironment and are involved peritoneal metastasis in gastric cancer
Abstract
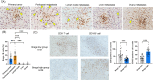
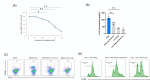
Peritoneal metastasis (PM) of gastric cancer (GC) has an immune escape environment. Regulatory B cells (Bregs), characterized by IL-10 production, play an important role in the tumor immunity; however, the function of Bregs in PM remains unclear. We investigated the frequency and effects of Bregs on other immune cells in the PM using clinical specimens and mouse models of PM. In the peripheral blood and ascites, Breg frequency was significantly higher in patients with GC with PM than in those without PM. In clinical PM samples, Breg frequency was an independent prognostic factor. In the mouse PM model, peritoneal tumors showed higher Breg infiltration than subcutaneous tumors. In the PTEN-deficient PM model, activation of Bregs promoted ascites and peritoneal tumor growth, decreased the infiltration of CD8+ T cells, and increased the infiltration of M2 macrophages. In contrast, treatment with wortmannin, a phosphatidylinositol 3-kinase (PI3K) inhibitor, suppressed Breg infiltration, resulting in decreased M2 macrophage infiltration and increased CD8+ T cell infiltration. Bregs are indicated to be involved in immunosuppression of PM and are promising targets for improving the efficacy of immunotherapy against PM.
Keywords: Gastric cancer; PI3K/Akt; Peritoneal metastasis; Regulatory B cell; Wortmannin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





References
-
- Shirao, K. et al. Randomized phase III study of 5-fluorouracil continuous infusion vs. sequential methotrexate and 5-fluorouracil therapy in Far advanced gastric cancer with peritoneal metastasis (JCOG0106). Jpn J. Clin. Oncol.43, 972–980. 10.1093/jjco/hyt114 (2013). - PubMed
-
- Wilke, H. et al. Ramucirumab plus Paclitaxel versus placebo plus Paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol.15, 1224–1235. 10.1016/s1470-2045(14)70420-6 (2014). - DOI - PubMed
-
- Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London England). 398, 27–40. 10.1016/s0140-6736(21)00797-2 (2021). - PMC - PubMed
-
- Kang, Y. K. et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.23, 234–247. 10.1016/s1470-2045(21)00692-6 (2022). - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

