Perspectives on mitochondrial dysfunction in the regeneration of aging skeletal muscle
- PMID: 40721743
- PMCID: PMC12305941
- DOI: 10.1186/s11658-025-00771-1
Perspectives on mitochondrial dysfunction in the regeneration of aging skeletal muscle
Abstract
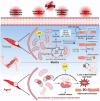
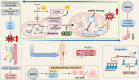
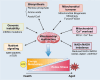
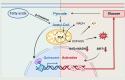
As the global population trends toward aging, the number of individuals suffering from age-related debilitating diseases is increasing. With advancing age, skeletal muscle undergoes progressive oxidative stress infiltration, coupled with detrimental factors such as impaired protein synthesis and mitochondrial DNA (mtDNA) mutations, culminating in mitochondrial dysfunction. Muscle stem cells (MuSCs), essential for skeletal muscle regeneration, also experience functional decline during this process, leading to irreversible damage to muscle integrity in older adults. A critical contributing factor is the loss of mitochondrial metabolism and function in MuSCs within skeletal muscle. The mitochondrial quality control system plays a pivotal role as a modulator, counteracting aging-associated abnormalities in energy metabolism and redox imbalance. Mitochondria meet functional demands through processes such as fission, fusion, and mitophagy. The significance of mitochondrial morphology and dynamics in the mechanisms of muscle regeneration has been consistently emphasized. In this review, we provide a comprehensive summary of recent advances in understanding the mechanisms of aging-related mitochondrial dysfunction and its role in hindering skeletal muscle regeneration. Additionally, we present novel insights into therapeutic approaches for treating aging-related myopathies.
Keywords: Aging; Mitochondrial dynamics; Mitophagy; Oxidative stress; Skeletal muscle regeneration.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no potential conflicts of interest.
Figures
References
-
- Seale P, Asakura A, Rudnicki MA. The potential of muscle stem cells. Dev Cell. 2001;1(3):333–42. 10.1016/s1534-5807(01)00049-1. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

