Subnational introduction of the RTS,S/AS01E malaria vaccine into routine immunization: experience and lessons from the three pilot countries
- PMID: 40721790
- PMCID: PMC12305954
- DOI: 10.1186/s12936-025-05484-6
Subnational introduction of the RTS,S/AS01E malaria vaccine into routine immunization: experience and lessons from the three pilot countries
Abstract
Background: In October 2021, the World Health Organization (WHO) recommended the RTS,S/AS01E (RTS,S) malaria vaccine for the prevention of Plasmodium falciparum malaria in children living in endemic areas informed by evidence from the subnational pilot introduction and evaluation in Ghana, Kenya, and Malawi as part of the WHO-coordinated Malaria Vaccine Implementation Programme (MVIP). With the global vaccine supply boosted by the pre-qualification of a second malaria vaccine, R21/Matrix-M (R21), in October 2023, many endemic countries (20 as of April 2025) have introduced malaria vaccines into their national childhood immunization and malaria control programmes. More endemic countries are expected to introduce or scale up malaria vaccines in 2025 and beyond. This paper summarizes key operational lessons from the pilot countries to facilitate the introduction and scale-up of malaria vaccination in other countries.
Methods: Pilot areas were identified, in part, based on local malaria epidemiology. RTS,S was initially introduced in randomly selected areas, while other areas served as comparators until the four-dose schedule vaccine was scaled up following the WHO recommendation in 2021. In Ghana and Kenya, the vaccine was administered at ages 6, 7, 9, and 24 months (Ghana switched to administer the fourth dose at age 18 months in 2023), and Malawi chose a schedule of 5, 6, 7, and 22 months.
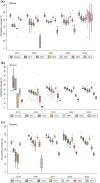
Results: Vaccination coverage improved over time, reaching about 80% for the first dose and around 75% for the third dose by 2023 in the initial pilot areas. Implementation challenges included an inadequate understanding of age eligibility among healthcare workers during the early phase of introduction, low fourth dose coverage (with a median coverage of 46% in 2023 across the three countries), and disruptions to service delivery caused by disease outbreaks and other natural disasters. Health stakeholders and caregivers attested to the positive impact of introducing the malaria vaccine, including a reduction in malaria hospitalizations and the strengthening of the National Immunization Programme (NIP) through routine immunization refresher training and supportive supervision.
Conclusions: The pilot highlighted lessons for malaria vaccine introduction: (1) clearly outlined roles and responsibilities of key stakeholders including NIP and National Malaria Programme (NMP); (2) appropriate approach to vaccine introduction launch, communication, and demand generation to enhance vaccine uptake; (3) flexibility with dose scheduling to optimize coverage; and (4) updated data collection tools for accurate documentation, and data quality.
Keywords: Ghana; Kenya; Malaria Vaccine Implementation Programme; Malaria prevention; Malaria vaccine; Malaria vaccine coverage; Malawi; New vaccine introduction; Pilot implementation; RTS,S/AS01.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Informed consent: Informed consent was not required for the pilot implementation, as the RTS, Svaccine was introduced into the national immunization programmes in the three countries.Consent was, however, obtained for the independent evaluation studies, which are publishedseparately.
Figures

References
-
- WHO. World Malaria Report 2024. Geneva: World Health Organization; 2024.
-
- Asante KP, Mathanga DP, Milligan P, Akech S, Oduro A, Mwapasa V, et al. Feasibility, safety, and impact of the RTS, S/AS01E malaria vaccine when implemented through national immunisation programmes: evaluation of cluster-randomised introduction of the vaccine in Ghana, Kenya, and Malawi. Lancet. 2024;403:1660–70. - PMC - PubMed
-
- European Medicines Agency. First malaria vaccine receives positive scientific opinion from EMA 2015. https://www.ema.europa.eu/en/news/first-malaria-vaccine-receives-positiv.... Accessed 7 July 2025.
-
- World Health Organization. Full evidence report on the RTS,S/AS01 malaria vaccine. Geneva, World Health Organization. 2021. https://cdn.who.int/media/docs/default-source/immunization/mvip/full-evi.... Accessed 8 July 2025.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

