Xanthosine alleviates myocardial ischemia-reperfusion injury through attenuation of cardiomyocyte ferroptosis
- PMID: 40722060
- PMCID: PMC12306105
- DOI: 10.1186/s11658-025-00766-y
Xanthosine alleviates myocardial ischemia-reperfusion injury through attenuation of cardiomyocyte ferroptosis
Abstract
Background: Ischemic heart disease remains a leading cause of morbidity and mortality worldwide, with myocardial ischemia-reperfusion (I/R) injury significantly contributing to cardiomyocyte death and poor outcomes post-acute myocardial infarction (AMI). Emerging evidence highlights metabolic changes during myocardial injury, particularly in purine metabolism. This study investigates the protective role of xanthosine (XTS), a purine metabolism intermediate, in alleviating I/R injury.
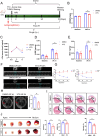
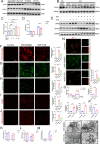
Methods: Neonatal and adult mouse myocardial tissues post-myocardial infarction (MI) were analyzed using untargeted and targeted metabolomics to explore metabolic profiles. The effects of XTS on I/R injury were evaluated in vivo using a murine I/R model and in vitro with hypoxia/reoxygenation-treated neonatal rat cardiomyocytes (NRCMs). Cardiac function, fibrosis, apoptosis, oxidative stress markers, and ferroptosis-related pathways were assessed via echocardiography, biochemical assays, western blotting, and electron microscopy. Integrated drug affinity responsive target stability (DARTS)-based drug target screening and RNA-seq transcriptomic profiling elucidate XTS-mediated mechanisms against I/R injury.
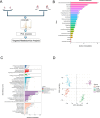
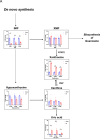
Results: Metabolomics revealed distinct differences in purine metabolism between neonatal and adult mice post-MI, with significant XTS accumulation observed in neonatal hearts. In vivo, XTS treatment in adult mice enhanced left ventricular function, reduced fibrosis, and alleviated lipid peroxidation and mitochondrial damage post-I/R injury. In vitro, XTS significantly improved cardiomyocyte viability, reduced oxidative stress, and mitigated ferroptosis by restoring glutathione peroxidase 4 (GPX4) levels and reducing acyl-coenzyme A synthetase long-chain family member 4 (ACSL4) expression. Mechanistically, XTS stabilized metabolic enzymes, upregulated L-arginine and glutathione (GSH) to mitigate reactive oxygen species(ROS), and inhibited ferroptosis.
Conclusions: XTS, a key purine metabolism intermediate, improves cardiac remodeling and function following I/R injury by suppressing ferroptosis and reducing mitochondrial ROS production. These findings provide novel insights into the therapeutic potential of XTS as an adjunctive treatment for patients with AMI undergoing revascularization.
Keywords: Ferroptosis; Ischemia–reperfusion (I/R) Injury; Purine metabolism; Reactive oxygen species (ROS); Xanthosine (XTS).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal procedures were approved by the Animal Ethics Committee of Southeast University (no. 20240219008, 19 February 2024). All of the experiments about animals were performed in accordance with the Basel Declaration. Animal Ethics Committee of Southeast University strictly adheres to the principles and guidelines established by the International Council for Laboratory Animal Science (ICLAS) to ensure that our animal research meets international ethical standards. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures





References
-
- Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–639. - PubMed
-
- Lopaschuk GD, Collins-Nakai RL, Itoi T. Developmental changes in energy substrate use by the heart. Cardiovasc Res. 1992;26(12):1172–80. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

