Targeting CD276 with Adapter-CAR T-cells provides a novel therapeutic strategy in small cell lung cancer and prevents CD276-dependent fratricide
- PMID: 40722088
- PMCID: PMC12305915
- DOI: 10.1186/s13045-025-01729-8
Targeting CD276 with Adapter-CAR T-cells provides a novel therapeutic strategy in small cell lung cancer and prevents CD276-dependent fratricide
Abstract
Background: Survival rates in Small Cell Lung Cancer (SCLC) remain dismal, posing a huge medical need for novel therapies. T-cells, engineered to express chimeric antigen receptors (CAR-T) have demonstrated clinical activity against a variety of haematological malignancies. Yet, efficacy against solid tumour entities remains limited.
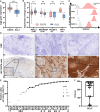
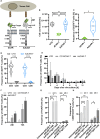
Methods: In this study, we investigated the expression of CD276 (B7-H3), an immune checkpoint molecule and promising target antigen for CAR-T therapy in SCLC, at the RNA and protein level. We further developed novel Fab-based adapter molecules (AM) targeting CD276 and optimized our previously established modular Adapter CAR-T (AdCAR-T) platform as well as AM dosing schemes.
Results: CD276 is broadly expressed across SCLC subtypes, representing a promising target for CAR-T therapy. We describe that T-cell activation and CAR-signalling induces CD276-expression on CAR-T, resulting in CD276-dependent fratricide, limiting anti-CD276-CAR-T expansion and activity. The AdCAR-T platform allows CAR-T expansion in absence of CD276 targeting. Novel CD276 targeted AMs demonstrate potent in vitro and in vivo activity against SCLC. Intermittent AM-dosing allows functional persistence of AdCAR-T in vivo in contrast to CD276-targeted conventional CAR-T. AdCAR-T in vivo expansion and activity is further promoted by introducing activation-induced, AM remote controlled, IL-18 secretion into the AdCAR-T design.
Conclusion: We identified CD276 as a promising target antigen, uniformly expressed in SCLC and demonstrate the therapeutic potential of novel anti-CD276 Fab-based AM in combination with optimized, IL-18 armoured AdCAR-T.
Keywords: AdCAR-T; CD276; Fab-based adapter molecule; Fratricide; Immunotherapy; In vivo solid tumor targeting; SCLC.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: For human FFPE samples informed consent was obtained from all patients or their guardians for use of their samples for research, as approved by the ethics committee of the University Hospital Tuebingen in accordance with to the Declaration of Helsinki (ethics approval No. 508-2016BO1). For the use of human blood informed consent was obtained from all patients and approved by the Institutional Ethical Review Board 761/2015BO2. All in vivo studies were carried out in accordance with the guidelines of the Federation of European Laboratory Animal Science Associations (FELASA) in the animal facilities of the University Children´s Hospital Tuebingen, Germany. Experiments were conducted according to the standards of the ethics committee board and animal care committee of the regional administrative council of Tuebingen (approval number K04/21G). Consent for publication: Not applicable. Competing interests: This research study was partially funded by Miltenyi Biotec B.V. & CO. KG, Bergisch Gladbach, Germany.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

