Chronic intermittent hypoxia impairs BM-MSC osteogenesis and long bone growth through regulating histone lactylation
- PMID: 40722170
- PMCID: PMC12306074
- DOI: 10.1186/s12967-025-06849-w
Chronic intermittent hypoxia impairs BM-MSC osteogenesis and long bone growth through regulating histone lactylation
Abstract
Background: Chronic intermittent hypoxia (CIH) caused by OSA often results in serious complications. However, the adverse effects of CIH on bone growth and development are often overlooked.
Methods: CIH intervention was conducted using an OxyCycler model A84 system for 8 h per day (from 8:00 a.m. to 4:00 p.m.) over a period of 4 weeks. Body and femur lengths were measured, and micro-CT, histological analysis, and ELISA were performed to evaluate femoral development. Metabolomic, single-cell transcriptomic, Western blot, and ChIP‒qPCR analyses were conducted to explore the potential mechanisms underlying CIH-induced inhibition of long bone growth. T0070907 was administered intraperitoneally (0.5 mg/kg) every two days to investigate its effect on long bone growth under CIH conditions.
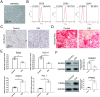
Results: Here, we showed that CIH stimulation during long bone development significantly inhibited long bone growth. Multiomics analysis revealed that CIH induces anaerobic glycolysis in bone marrow mesenchymal stem cells (BM-MSCs), promotes adipogenic differentiation, and reduces their osteogenic differentiation capacity. Mechanistic studies demonstrated that CIH-induced lactate accumulation enhances lactylation at histone H3 lysine 18 (H3K18) on the PPARγ promoter in BM-MSCs, leading to the transcriptional activation of PPARγ and a consequent imbalance between the adipogenic and osteogenic differentiation of BM-MSCs. The PPARγ inhibitor T0070907 could partially rescue long bone developmental disorders induced by CIH.
Conclusions: Our findings reveal an epigenetic mechanism underlying CIH-induced long bone dysplasia and highlight T0070907 as a promising targeted therapeutic agent.
Keywords: BM-MSCs; Bone development; Chronic intermittent hypoxia; Histone lactylation; PPARγ.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committee of Shanghai Sixth People’s Hospital (No. 2024 − 0632). Consent for publication: Not applicable. Competing interests: The authors confirm that there are no conflicts of interest.
Figures





Similar articles
-
[Yougui Yin attenuates adipogenic differentiation of bone marrow mesenchymal stem cells by modulating PPARγ pathway to treat glucocorticoid-induced osteonecrosis].Zhongguo Zhong Yao Za Zhi. 2025 Jun;50(12):3356-3367. doi: 10.19540/j.cnki.cjcmm.20250307.401. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40686113 Chinese.
-
Mechanical stimulation-induced purinome priming fosters osteogenic differentiation and osteointegration of mesenchymal stem cells from the bone marrow of post-menopausal women.Stem Cell Res Ther. 2024 Jun 18;15(1):168. doi: 10.1186/s13287-024-03775-4. Stem Cell Res Ther. 2024. PMID: 38886849 Free PMC article.
-
Identification of a Novel Gene ARNT2 for Osteogenic Differentiation of Mesenchymal Stem Cells.Calcif Tissue Int. 2025 Jul 18;116(1):100. doi: 10.1007/s00223-025-01407-4. Calcif Tissue Int. 2025. PMID: 40679635 Free PMC article.
-
Corticosteroids for the treatment of Duchenne muscular dystrophy.Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4. Cochrane Database Syst Rev. 2016. PMID: 27149418 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Vanek J, Prasko J, Genzor S, Ociskova M, Kantor K, Holubova M, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–8. 10.1016/j.sleep.2020.03.017 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

