Unmet needs in hereditary angioedema: an international survey of physicians
- PMID: 40722187
- PMCID: PMC12306030
- DOI: 10.1186/s13023-025-03739-8
Unmet needs in hereditary angioedema: an international survey of physicians
Abstract
Background: Hereditary angioedema (HAE) is a rare and potentially life-threatening genetic disorder characterized by unpredictable attacks of angioedema. MENTALIST (UnMEt Needs in herediTAry angioedema-a gLobal physIcian perSpecTive) is the first international survey uncovering unmet needs and identifying barriers to optimal management in HAE following the latest update of the World Allergy Organization (WAO)/European Academy of Allergy and Clinical Immunology (EAACI) HAE guidelines.
Methods: This web-based survey comprised 24 questions on HAE management and unmet needs. HAE-expert physicians from the Angioedema Centers of Reference and Excellence network ranked unmet needs according to their own perspectives and their patients' perspectives, using a 10-point Likert scale ranging from 0 (not a challenge/unmet need at all) to 10 (huge challenge/unmet need).
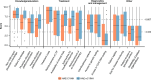
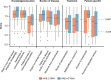
Results: Of 64 respondents from 32 countries, most (91%) had > 5 years of experience in managing HAE. Overall, 48% of respondents (n = 31/64) reported that < 50% of their patients had achieved the WAO/EAACI HAE treatment goals of total disease control and "normalization" of life at the time of the survey. Implementation of consensus recommendations was found to be inconsistent across regions. Gaps in non-HAE-expert physician knowledge, treatment costs, and reimbursement for long-term prophylaxis were the highest-priority challenges according to the respondents. Burden of disease remains a challenge among patients, as reported by their physicians.
Conclusions: The MENTALIST findings highlight a need for removal of barriers to HAE treatment goals and propose a call to action to improve access to treatments, for greater provision of education for physicians and patients, critical collaboration with patient organizations and industry stakeholders and ultimately to optimize HAE care.
Keywords: Guidelines; Hereditary angioedema; Management; Treatment goals; Unmet needs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: TB was a speaker for, and/or advisor for, and/or has received research funding from Almirall, Aquestive, BioCryst, CSL Behring, GSK, Hexal, KalVista Pharmaceuticals, Medac, Novartis, Pharming, Roche, Sanofi-Aventis, Swixx BioPharma, and Takeda. FA declares no conflict of interest in relation to this work. AA reports conference travel support and/or honoraria from BioCryst, Covis Pharma, CSL Behring, GSK, and Takeda; and clinical trial support from Astria, BioCryst, Ionis Pharmaceuticals, KalVista Pharmaceuticals, Octapharma, Pharvaris, and Takeda. CVA is a clinical trial/registry investigator for BioCryst, CSL Behring, Intellia, Novartis, Pharvaris, and Takeda. MAA-N declares no conflict of interest in relation to this work. SA declares no conflict of interest in relation to this manuscript. SA has conducted studies, and was an advisor and speaker, for ALK, Allakos, AstraZeneca, BioCryst, Blueprint, CSL Behring, Leo Pharma, Moxie, Novartis, Pharvaris, Sanofi, Takeda, and Thermo Fisher. MA declares no conflict of interest in relation to this work. MA-A received speaker and advisory board honoraria from AstraZeneca, GSK, and Sanofi. RMA declares no conflict of interest in relation to this work. AB has participated as a speaker for Takeda, and in advisory boards for CSL Behring and Pint Pharma. IB-G is or recently was a speaker, advisor, and engaged in research and educational projects for, and/or received research and consultancy grants from, BioCryst, CSL Behring, KalVista Pharmaceuticals, Novartis, Pharming, Pharvaris, and Takeda. LBo has consulted/served as speaker for, engaged in research and educational projects with, or accepted travel grants from BioCryst, Blueprint, CSL Behring, GSK, KalVista Pharmaceuticals, Novartis, Pharvaris, and Takeda. LBr declares no conflict of interest in relation to this work. MB has received travel and congress bursaries and lecture fees from Pharming and Takeda. PJB declares no conflict of interests in relation to this work. PB has received consultancy grants from BioCryst, CSL Behring, Intellia, and Takeda. SDB has received speaker/advisor fees and/or research funding from Astria, Canadian Blood Services, CSL Behring, Green Cross, Grifols, Ionis Pharmaceuticals, KalVista Pharmaceuticals, Novartis, Octapharma, Pharvaris, Sanofi, Takeda, and WSIB. HC-N has received grants for consultation from CSL Behring, Pint Pharma, and Takeda, and has received speaker grants from Takeda. OCL declares no conflict of interest in relation to this work. TC is a speaker for Astria Therapeutics, BioMarin, CSL Behring, Grifols, Regeneron, and Takeda; and has received research and consultancy grants from Astria, CSL Behring, BioCryst, BioMarin, GSK, Intellia, Ionis, KalVista Pharmaceuticals, Pharvaris, and Takeda. TC is a member of the US Hereditary Angioedema Association Medical Advisory Board and Director of the ACARE International Angioedema Center at Penn State University in Hershey, PA, USA. ADD has received honoraria from BioCryst, CSL Behring, and Takeda for advisory board and speaking services; has received reasonable expenses to attend meetings and conferences from BioCryst and Pharming; and has been a sub-investigator for clinical trials sponsored by BioCryst Pharmaceuticals, Ionis Pharmaceuticals, and KalVista Pharmaceuticals. SDDJr has received payment or honoraria from AstraZeneca, Chiesi, and Novartis. SDDJr received support for attending meetings and/or travel from CSL Behring, Sanofi, and Takeda. DF declares no conflict of interest in relation to this work. HF has received research grants from CSL Behring, Pharming, and Takeda; served as an advisor for BioCryst, CSL Behring, KalVista Pharmaceuticals, Intellia, Ionis Pharmaceuticals, ONO Pharmaceutical, Pharming, and Takeda; and participated in clinical trials/registries for BioCryst, CSL Behring, KalVista Pharmaceuticals, Pharming, Pharvaris, and Takeda. JSF has received speaker and/or consultancy fees for presentations and advisory board participation from CSL Behring, Menarini, Novartis, Takeda, and Viatris. ASG has received research funding from Brazilian Entity of Research (CNPq) and Takeda/Shire; and fees for educational activities or has acted as a consultant for Catalyst Pharmaceuticals, CSL Behring, KalVista Pharmaceuticals, MultiCare, Pharvaris, Pint Pharma, and Takeda. JG was a speaker and/or advisor for, and/or has received research funding from, BioCryst, CSL Behring, KalVista Pharmaceuticals, and Takeda. MGu has received honoraria for educational purposes from CSL Behring, Novartis, and Takeda; participated in advisory boards organized by CSL Behring, Novartis, and Takeda; and has received funding to attend conferences and educational events from CSL Behring, Novartis, Pharming, and Takeda. MGu is a clinical trial/registry investigator for BioCryst, CSL Behring, Novartis, Pharming, Pharvaris, and Takeda; and is a researcher from the VHIR program for promoting research activities. MGo has received honoraria for educational purposes or medical advice from AbbVie, AstraZeneca, Leo Pharma, Lilly, Novartis, Pfizer, Sanofi, and Takeda. VG-P has participated in clinical trials/registries for BioCryst, CSL Behring, KalVista Pharmaceuticals, Pharming, and Takeda. MH has received speaker/consultancy fees from BioCryst, CSL Behring, KalVista Pharmaceuticals, Pharvaris, and Takeda. RH has received speaker/consultancy fees and travel grants from, and/or participated in advisory boards for, CSL Behring, Pharming, Shire, and Takeda; and has served as a Principal Investigator for clinical trials sponsored by BioCryst Pharmaceuticals, CSL Behring, KalVista Pharmaceuticals, Pharming, and Pharvaris Netherlands. AJ declares no conflict of interest in relation to this work. CHK has received speaker/consultancy fees for presentations and advisory board participation from CSL Behring, KalVista Pharmaceuticals, Pharvaris, and Takeda. SK declares no conflict of interest in relation to this work. TK is or recently was a speaker and/or advisor for, and/or has received research funding from, BioCryst, Blueprint, CSL Behring, HAL Allergy, KalVista Pharmaceuticals, Kiniksa, Novartis, Pharvaris, Sanofi-Aventis, and Takeda/Shire. EL is or recently was a speaker and/or advisor for, and/or has received research funding from, CSL Behring, Generium, Novartis, Octapharma, and Takeda/Shire. JILS declares no conflict of interest in relation to this work. RLB has received speaker/consultancy fees from, and/or participated in advisory boards for, BioCryst, CSL Behring, Novartis, Pharming, and Takeda; and is/has been a clinical trial/registry investigator for BioCryst, Ionis, KalVista Pharmaceuticals, Pharvaris, and Takeda. HM declares no conflict of interest in relation to this work. MMet has received honoraria as a speaker and/or advisor from AbbVie, ALK-Abelló, Almirall, Amgen, Argenx, AstraZeneca, Bayer, Beiersdorf, Celldex, Celltrion, Escient, Galderma, GSK, Incyte, Jasper, Novartis, Pfizer, Pharvaris, Regeneron, Sanofi, Teva, Third Harmonic Bio, and Vifor. IN is or recently was a speaker for Sanofi and Takeda. NTM declares no conflict of interest in relation to this work. SN declares no conflict of interest in relation to this work. CASP declares no conflict of interest in relation to this work. GP has received speaker fees, and/or consultancy fees, and/or travel support from CSL Behring, Swixx BioPharma, and Takeda. JP is or recently was a speaker and/or advisor for, and/or has received research funding from, Astria, BioCryst, CSL Behring, Glenmark, Novartis, Pharming, Pharvaris, Sanofi/Regeneron, and Takeda. MPLF declares no conflict of interest in relation to this work. NRF declares no conflict of interest in relation to this work. BES declares no conflict of interest in relation to this work. FSS reports personal fees and other from AstraZeneca, CSL Behring, GSK, Novartis, and Takeda. MS has received grants and/or fees as a speaker from BioCryst, CSL Behring, KalVista Pharmaceuticals, Pharming, and Takeda; and is a clinical trial investigator for BioCryst, Ionis, Isis, KalVista Pharmaceuticals, and Pharvaris. ST was a speaker and/or advisor for CSL Behring and Takeda; and participated in clinical trials/registries for BioCryst, CSL Behring, Ionis Pharmaceuticals, and Takeda. AV has received honoraria for educational lectures, consultancy, sponsorship for educational meetings, and research projects from AstraZeneca, Astria Pharmaceuticals, Berlin-Chemie/Menarini Group, CSL Behring, Ewopharma, Ionis, KalVista Pharmaceuticals, Novartis, Organon, Pharming Group N. V., Pharvaris, Takeda/Shire, SOBI, Stallergenes Greer, and Teva. CW has received honoraria for scientific lectures from Abbott, AstraZeneca, GSK, Menarini, Novartis, Sanofi, and Takeda; and research support from Abbott and Sanofi. JCYW declares no conflict of interest in relation to this work. EY declares no conflict of interest in relation to this work. YL is a full-time employee of CSL Behring LLP and shareholder of CSL Limited. CN is a full-time employee of CSL Behring AG and shareholder of CSL Limited. MMau was a speaker and/or advisor for, and/or received research funding from, Astria, BioCryst, CSL Behring, KalVista Pharmaceuticals, Pharvaris, and Takeda. MMag has received financial support from CSL Behring for acting as a study center investigator during the conduct of the study and personal fees from BioCryst, CSL Behring, KalVista Pharmaceuticals, Novartis, Octapharma, Pharming Technologies, and Takeda/Shire. PHL was a speaker and/or advisor for, and/or has received research funding from, CSL Behring, KalVista Pharmaceuticals, Pharvaris, and Takeda.
Figures


References
-
- Maurer M, Magerl M, Betschel S, Aberer W, Ansotegui IJ, Aygoren-Pursun E, et al. The international WAO/EAACI guideline for the management of hereditary angioedema–the 2021 revision and update. Allergy. 2022;77(7):1961–90. - PubMed
-
- Lumry WR, Settipane RA. Hereditary angioedema: epidemiology and burden of disease. Allergy Asthma Proc. 2020;41(Suppl 1):S08-S13. - PubMed
-
- Reshef A, Buttgereit T, Betschel SD, Caballero T, Farkas H, Grumach AS, et al. Definition, acronyms, nomenclature, and classification of angioedema (DANCE): AAAAI, ACAAI, ACARE, and APAAACI DANCE consensus. J Allergy Clin Immunol. 2024;154(2):398–411. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

