Competitive roles of slow/delta oscillation-nesting-mediated sleep disruption under acute methamphetamine exposure in monkeys
- PMID: 40722246
- PMCID: PMC12303792
- DOI: 10.1631/jzus.B2400048
Competitive roles of slow/delta oscillation-nesting-mediated sleep disruption under acute methamphetamine exposure in monkeys
Abstract
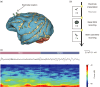
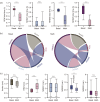
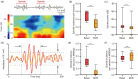
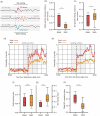
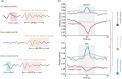
Abuse of amphetamine-based stimulants is a primary public health concern. Recent studies have underscored a troubling escalation in the inappropriate use of prescription amphetamine-based stimulants. However, the neurophysiological mechanisms underlying the impact of acute methamphetamine exposure (AME) on sleep homeostasis remain to be explored. This study employed non-human primates and electroencephalogram (EEG) sleep staging to evaluate the influence of AME on neural oscillations. The primary focus was on alterations in spindles, delta oscillations, and slow oscillations (SOs) and their interactions as conduits through which AME influences sleep stability. AME predominantly diminishes sleep-spindle waves in the non-rapid eye movement 2 (NREM2) stage, and impacts SOs and delta waves differentially. Furthermore, the competitive relationships between SO/delta waves nesting with sleep spindles were selectively strengthened by methamphetamine. Complexity analysis also revealed that the SO-nested spindles had lost their ability to maintain sleep depth and stability. In summary, this finding could be one of the intrinsic electrophysiological mechanisms by which AME disrupted sleep homeostasis.
苯丙胺类兴奋剂的滥用是全球重要的公共健康风险之一。近期研究指出,处方安非他命类药物的滥用呈现显著上升趋势。然而,急性甲基苯丙胺暴露(AME)影响睡眠稳态的神经生理机制仍有待探索。本研究采用非人灵长类动物(恒河猴)为模型,利用脑电图(EEG)睡眠分期的方法评估AME对神经振荡的调控作用,并重点研究AME对睡眠纺锤波、delta波和慢波(SO)的差异性影响,及其在睡眠稳定性调控中的相互作用机制。AME显著抑制非快速眼动2期(NREM2)的睡眠纺锤波,并对SO和delta波具有差异性调控作用。此外,甲基苯丙胺特异性增强了SO和delta波与睡眠纺锤波的嵌套关系;复杂度特征分析发现,SO嵌套的纺锤波维持睡眠深度和稳定性的生理功能也出现显著损伤。上述结果在神经振荡网络层面上阐述了AME导致睡眠稳态破坏的内在电生理机制。.
苯丙胺类兴奋剂的滥用是全球重要的公共健康风险之一。近期研究指出,处方安非他命类药物的滥用呈现显著上升趋势。然而,急性甲基苯丙胺暴露(AME)影响睡眠稳态的神经生理机制仍有待探索。本研究采用非人灵长类动物(恒河猴)为模型,利用脑电图(EEG)睡眠分期的方法评估AME对神经振荡的调控作用,并重点研究AME对睡眠纺锤波、delta波和慢波(SO)的差异性影响,及其在睡眠稳定性调控中的相互作用机制。AME显著抑制非快速眼动2期(NREM2)的睡眠纺锤波,并对SO和delta波具有差异性调控作用。此外,甲基苯丙胺特异性增强了SO和delta波与睡眠纺锤波的嵌套关系;复杂度特征分析发现,SO嵌套的纺锤波维持睡眠深度和稳定性的生理功能也出现显著损伤。上述结果在神经振荡网络层面上阐述了AME导致睡眠稳态破坏的内在电生理机制。
Keywords: Addiction; Amphetamine; Delta oscillation; Electroencephalogram (EEG); Sleep stage; Slow oscillation (SO).
Figures


Similar articles
-
Interrelations and functional roles of key oscillatory activities during daytime sleep in older adults.J Sleep Res. 2024 May;33(3):e13981. doi: 10.1111/jsr.13981. Epub 2023 Jul 24. J Sleep Res. 2024. PMID: 37488062
-
Sleep-slow oscillation-spindle coupling precedes spindle-ripple coupling during development.Sleep. 2024 May 10;47(5):zsae061. doi: 10.1093/sleep/zsae061. Sleep. 2024. PMID: 38452190
-
Timing of Methamphetamine Exposure during Adolescence Differentially Influences Parvalbumin and Perineuronal Net Immunoreactivity in the Medial Prefrontal Cortex of Female, but Not Male, Rats.Dev Neurosci. 2025;47(1):27-39. doi: 10.1159/000538608. Epub 2024 Mar 28. Dev Neurosci. 2025. PMID: 38547851 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Non-pharmacological interventions for sleep promotion in hospitalized children.Cochrane Database Syst Rev. 2022 Jun 15;6(6):CD012908. doi: 10.1002/14651858.CD012908.pub2. Cochrane Database Syst Rev. 2022. PMID: 35703367 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 82271515/the National Natural Science Foundation of China
- 2019015/the SJTU Trans-Med Awards Research
- KY20211478/the Scientific and Technological Innovation Action Plan of Shanghai
- 2021SHZDZX/the Shanghai Municipal Science and Technology Major Project
- SJTUHLXK2022/the Nursing Development Program of Shanghai Jiao Tong University School of Medicine
LinkOut - more resources
Full Text Sources
Medical

