The APOE-Microglia Axis in Alzheimer's Disease: Functional Divergence and Therapeutic Perspectives-A Narrative Review
- PMID: 40722268
- PMCID: PMC12293602
- DOI: 10.3390/brainsci15070675
The APOE-Microglia Axis in Alzheimer's Disease: Functional Divergence and Therapeutic Perspectives-A Narrative Review
Abstract
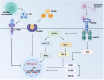
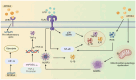
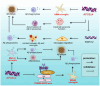
Apolipoprotein E (APOE) alleles play distinct roles in the pathogenesis of Alzheimer's disease (AD), with APOEε4 being the strongest genetic risk factor for late-onset AD, while APOEε2 appears protective. Despite extensive research, the precise mechanisms by which APOE alleles contribute to AD pathology remain incompletely understood. Recent advances in multi-omics technologies and single-cell analyses have revealed that APOE alleles shape microglial phenotypes, thereby affecting amyloid clearance, inflammatory responses, tau pathology, and lipid metabolism. In this review, we provide a detailed overview of how APOE alleles differentially regulate microglial activation, inflammatory signaling, phagocytic activity, and lipid metabolism in the context of AD, with a particular focus on the APOEε4-mediated disruption of microglial homeostasis via pathways such as TREM2 signaling, NF-κB/NLRP3 activation, ACSL1 upregulation, and HIF-1α induction. These insights not only advance our understanding of APOE allele-specific contributions to AD pathology, but also highlight novel therapeutic strategies targeting the APOE-microglia axis.
Keywords: Alzheimer’s disease; apolipoprotein E; inflammation; lipid metabolism; microglia; phagocytosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
APOE Christchurch enhances a disease-associated microglial response to plaque but suppresses response to tau pathology.Mol Neurodegener. 2025 Jan 22;20(1):9. doi: 10.1186/s13024-024-00793-x. Mol Neurodegener. 2025. PMID: 39844286 Free PMC article.
-
APOE Christchurch enhances a disease-associated microglial response to plaque but suppresses response to tau pathology.bioRxiv [Preprint]. 2024 Jun 4:2024.06.03.597211. doi: 10.1101/2024.06.03.597211. bioRxiv. 2024. Update in: Mol Neurodegener. 2025 Jan 22;20(1):9. doi: 10.1186/s13024-024-00793-x. PMID: 38895362 Free PMC article. Updated. Preprint.
-
The Role of APOA-I in Alzheimer's Disease: Bridging Peripheral Tissues and the Central Nervous System.Pharmaceuticals (Basel). 2025 May 25;18(6):790. doi: 10.3390/ph18060790. Pharmaceuticals (Basel). 2025. PMID: 40573187 Free PMC article. Review.
-
CX3CL1 Pathway as a Molecular Target for Treatment Strategies in Alzheimer's Disease.Int J Mol Sci. 2023 May 4;24(9):8230. doi: 10.3390/ijms24098230. Int J Mol Sci. 2023. PMID: 37175935 Free PMC article. Review.
-
Selective reduction of astrocyte apoE3 and apoE4 strongly reduces Aβ accumulation and plaque-related pathology in a mouse model of amyloidosis.Mol Neurodegener. 2022 Feb 2;17(1):13. doi: 10.1186/s13024-022-00516-0. Mol Neurodegener. 2022. PMID: 35109920 Free PMC article.
References
-
- Rogus-Pulia N., Foundas A.L., Mueller K.D. Alzheimer’s Disease. In: Weissbrod P.A., Francis D.O., editors. Neurologic and Neurodegenerative Diseases of the Larynx. Springer International Publishing; Cham, Switzerland: 2020. pp. 177–189. - DOI
-
- Dumurgier J., Sabia S. Epidemiology of Alzheimer’s disease: Latest trends. Rev. Prat. 2020;70:149–151. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

