Sending the Signal to Bone: How Tumor-Derived EVs Orchestrate Pre-Metastatic Niche Formation and Skeletal Colonization
- PMID: 40722712
- PMCID: PMC12292996
- DOI: 10.3390/biomedicines13071640
Sending the Signal to Bone: How Tumor-Derived EVs Orchestrate Pre-Metastatic Niche Formation and Skeletal Colonization
Abstract
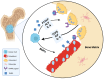
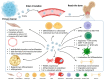
Bone is a preferred site for disseminated tumor cells, yet the molecular mechanisms that prepare the skeletal microenvironment for metastatic colonization are only beginning to be understood. At the heart of this process are extracellular vesicles (EVs), nano-sized, lipid-encapsulated particles secreted by cancer cells and stromal components. This review consolidates current findings that position EVs as key architects of the bone-metastatic niche. We detail the biogenesis of EVs and their organotropic distribution, focusing on how integrin patterns and bone-specific ligands guide vesicle homing to mineralized tissues. We then outline the sequential establishment of the pre-metastatic niche, driven by EV-mediated processes including fibronectin deposition, stromal cell reprogramming, angiogenesis, neurogenesis, metabolic reconfiguration, and immune modulation, specifically, the expansion of myeloid-derived suppressor cells and impaired lymphocyte function. Within the bone microenvironment, tumor-derived EVs carrying microRNAs and proteins shift the balance toward osteoclastogenesis, inhibit osteoblast differentiation, and disrupt osteocyte signaling. These alterations promote osteolytic destruction or aberrant bone formation depending on tumor type. We also highlight cutting-edge imaging modalities and single-EV omics technologies that resolve EV heterogeneity and identify potential biomarkers detectable in plasma and urine. Finally, we explore therapeutic approaches targeting EVs, such as inhibition of nSMase2 or Rab27A, extracorporeal EV clearance, and delivery of engineered, bone-targeted vesicles, while addressing translational challenges and regulatory considerations. This review offers a roadmap for leveraging EV biology in predicting, preventing, and treating skeletal metastases by integrating advances across basic biology, bioengineering, and translational science.
Keywords: EV-targeted therapy; bone metastasis; extracellular vesicles; liquid biopsy biomarkers; osteoclastogenesis; pre-metastatic niche.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

Similar articles
-
"Small extracellular vesicles: messengers at the service of breast cancer agenda in the primary and distant microenvironments".J Exp Clin Cancer Res. 2025 Jul 21;44(1):216. doi: 10.1186/s13046-025-03471-y. J Exp Clin Cancer Res. 2025. PMID: 40691627 Free PMC article. Review.
-
Diverse Populations of Extracellular Vesicles with Opposite Functions during Herpes Simplex Virus 1 Infection.J Virol. 2021 Feb 24;95(6):e02357-20. doi: 10.1128/JVI.02357-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33361424 Free PMC article.
-
Extracellular vesicles from ovarian cancer cells induce senescent lipid-laden macrophages to facilitate omental metastasis.J Nanobiotechnology. 2025 Jul 26;23(1):540. doi: 10.1186/s12951-025-03612-7. J Nanobiotechnology. 2025. PMID: 40713812 Free PMC article.
-
Molecular engineering of extracellular vesicles for drug delivery: Strategies, challenges, and perspectives.J Control Release. 2025 Jul 26;386:114068. doi: 10.1016/j.jconrel.2025.114068. Online ahead of print. J Control Release. 2025. PMID: 40721069 Review.
-
Fibroblast proximity to a tumor impacts fibroblast extracellular vesicles produced by 3D bioprinted stromal models.Biomater Sci. 2025 Jul 8;13(14):3814-3827. doi: 10.1039/d4bm01569j. Biomater Sci. 2025. PMID: 40492327 Free PMC article.
References
-
- Huang J.-F., Shen J., Li X., Rengan R., Silvestris N., Wang M., Derosa L., Zheng X., Belli A., Zhang X.-L., et al. Incidence of Patients with Bone Metastases at Diagnosis of Solid Tumors in Adults: A Large Population-Based Study. Ann. Transl. Med. 2020;8:482. doi: 10.21037/atm.2020.03.55. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

