Efficacy of EA575 as an Antitussive and Mucoactive Agent in Preclinical In Vivo Models
- PMID: 40722746
- PMCID: PMC12292205
- DOI: 10.3390/biomedicines13071673
Efficacy of EA575 as an Antitussive and Mucoactive Agent in Preclinical In Vivo Models
Abstract
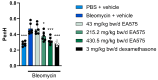
Background: The efficacy of EA575 in the treatment of respiratory diseases is described in various clinical studies, improving patients' disease-related symptoms. However, mechanistic in vivo data proving its beneficial effects are limited. Methods: Focusing on the treatment of acute airway inflammation and accompanying cough, this study aimed to elucidate antitussive and mucoactive properties of EA575, applying two animal models. Animals were treated orally twice daily for 7 days, resulting in 43, 215.2, or 430.5 mg/kg bw/d of EA575. Antitussive effects were investigated within an acute lung inflammation model of bleomycin-treated guinea pigs after citric acid exposure. Hereby, the number of coughs, enhanced pause (penH), and bronchoalveolar lavage fluid (BALF) were investigated. Mucoactivity of EA575 was assessed within a murine model, determining phenol red concentration in BALF. Results: EA575 treatment within the acute lung inflammation model reduced cough events up to 56% while reducing inflammatory cell influx in BALF dose-dependently, e.g., reducing neutrophils in BALF up to 70.9%. This suggests a strong connection between anti-inflammatory and antitussive properties of EA575. Furthermore, penH decreased in a dose-dependent manner, suggesting an ease in respiration. Mucoactivity was shown by a dose-dependent increase in phenol red concentration in BALF up to 38.9%. Notably, EA575/salbutamol co-administration resulted in enhanced phenol red secretion compared to respective single administrations. Conclusions: These data highlight the benefits of EA575 in treating cough-related respiratory diseases, particularly when accompanied by sputum, as EA575 has been shown to obtain mucoactivity. Furthermore, the combinatory effect of EA575/salbutamol treatment provides a foundation for future research in the treatment of chronic respiratory diseases.
Keywords: EA575; antitussive; cough; ivy leaf dry extract; mucoactive agent; respiratory disease.
Conflict of interest statement
Matthias Hufnagel, André Rademaekers, and Anika Weisert are all employed by Engelhard Arzneimittel GmbH & Co. KG, Herzbergstrasse 3, 61138 Niederdorfelden, Germany. Hanns Häberlein and Sebastian Franken declare that the research was conducted in the absence of any conflict of interest.
Figures




Similar articles
-
[Guidelines for the prevention and management of bronchial asthma (2024 edition)].Zhonghua Jie He He Hu Xi Za Zhi. 2025 Mar 12;48(3):208-248. doi: 10.3760/cma.j.cn112147-20241013-00601. Zhonghua Jie He He Hu Xi Za Zhi. 2025. PMID: 40050074 Chinese.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Positive expiratory pressure therapy versus other airway clearance techniques for bronchiectasis.Cochrane Database Syst Rev. 2017 Sep 27;9(9):CD011699. doi: 10.1002/14651858.CD011699.pub2. Cochrane Database Syst Rev. 2017. PMID: 28952156 Free PMC article.
-
Cilostazol for intermittent claudication.Cochrane Database Syst Rev. 2014 Oct 31;2014(10):CD003748. doi: 10.1002/14651858.CD003748.pub4. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2021 Jun 30;6:CD003748. doi: 10.1002/14651858.CD003748.pub5. PMID: 25358850 Free PMC article. Updated.
-
Pharmacological interventions for the prevention of bleeding in people undergoing elective hip or knee surgery: a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 16;1(1):CD013295. doi: 10.1002/14651858.CD013295.pub2. Cochrane Database Syst Rev. 2024. PMID: 38226724 Free PMC article.
References
-
- EMA . EMA/HMPC/325715/2017. Committee on Herbal Medicinal Products (HMPC); Amsterdam, the Netherlands: 2018. Assessment Report on Hedera helix L., Folium.
-
- Völp A., Schmitz J., Bulitta M., Raskopf E., Acikel C., Mösges R. Ivy Leaves Extract Ea 575 in the Treatment of Cough during Acute Respiratory Tract Infections: Meta-Analysis of Double-Blind, Randomized, Placebo-Controlled Trials. Sci. Rep. 2022;12:20041. doi: 10.1038/s41598-022-24393-1. - DOI - PMC - PubMed
-
- Greunke C., Hage-Hulsmann A., Sorkalla T., Keksel N., Haberlein F., Haberlein H. A Systematic Study on the Influence of the Main Ingredients of an Ivy Leaves Dry Extract on the Beta2-Adrenergic Responsiveness of Human Airway Smooth Muscle Cells. Pulm. Pharmacol. Ther. 2015;31:92–98. doi: 10.1016/j.pupt.2014.09.002. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

