Extracorporeal Cytokine Adsorption in Sepsis: Current Evidence and Future Perspectives
- PMID: 40722757
- PMCID: PMC12292210
- DOI: 10.3390/biomedicines13071684
Extracorporeal Cytokine Adsorption in Sepsis: Current Evidence and Future Perspectives
Abstract
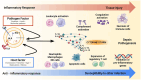
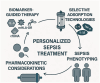
Background: Sepsis and septic shock are major contributors to global morbidity and mortality. The "cytokine storm," a hyper-inflammatory response, plays a central role in sepsis pathophysiology, leading to multi-organ failure. Extracorporeal cytokine adsorption therapies, such as CytoSorb, Toraymyxin, Oxiris, HA330/380, and Seraph 100 Microbind, aim to mitigate the inflammatory response by removing circulating cytokines and other mediators. Methods: A comprehensive search of Scopus and PubMed was conducted for studies published from January 2020 to May 2025. The search terms included "sepsis," "septic shock," and "extracorporeal cytokine adsorption." Relevant studies, including clinical trials and meta-analyses, were included to assess the efficacy and safety of these therapies. Results: Extracorporeal cytokine adsorption has shown promising results in reducing cytokine levels, improving organ function, and decreasing vasopressor requirements. However, evidence regarding mortality reduction remains inconsistent. Studies have demonstrated benefits in sepsis, ARDS, and cardiogenic shock, improving organ recovery and inflammatory markers. Conclusions: Extracorporeal cytokine adsorption is a potential adjunctive therapy in sepsis management, offering improvements in organ function and inflammatory control. While the mortality benefit remains uncertain, ongoing research and large-scale clinical trials are essential to define its clinical role and optimize its application.
Keywords: cytokine storm; extracorporeal cytokine adsorption; inflammation; mortality; sepsis; septic shock.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Meyer N.J., Prescott H.C. Sepsis and Septic Shock. N. Engl. J. Med. 2024;391:2133–2146. - PubMed
-
- Angus D.C., van der Poll T. Severe sepsis and septic shock. N. Engl. J. Med. 2013;369:840–851. - PubMed
-
- Schouten M., Wiersinga W.J., Levi M., van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J. Leukoc. Biol. 2008;83:536–545. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

