Targeting Cellular Senescence: Pathophysiology in Multisystem Age-Related Diseases
- PMID: 40722797
- PMCID: PMC12293066
- DOI: 10.3390/biomedicines13071727
Targeting Cellular Senescence: Pathophysiology in Multisystem Age-Related Diseases
Abstract
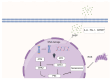
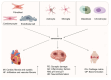
With the intensification of global aging, the incidence of age-related diseases (including cardiovascular, neurodegenerative, and musculoskeletal disorders) has been on the rise, and cellular senescence is identified as the core driving mechanism. Cellular senescence is characterized by irreversible cell cycle arrest, which is caused by telomere shortening, imbalance in DNA damage repair, and mitochondrial dysfunction, accompanied by the activation of the senescence-associated secretory phenotype (SASP). In this situation, proinflammatory factors and matrix-degrading enzymes can be released, thereby disrupting tissue homeostasis. This disruption of tissue homeostasis induced by cellular senescence manifests as characteristic pathogenic mechanisms in distinct disease contexts. In cardiovascular diseases, senescence of cardiomyocytes and endothelial cells can exacerbate cardiac remodeling. In neurodegenerative diseases, senescence of glial cells can lead to neuroinflammation, while in musculoskeletal diseases, it can result in the degradation of cartilage matrix and imbalance of bone homeostasis. This senescence-mediated dysregulation across diverse organ systems has spurred the development of intervention strategies. Interventional strategies include regular exercise, caloric restriction, senolytic drugs (such as the combination of dasatinib and quercetin), and senomorph therapies. However, the tissue-specific regulatory mechanisms of cellular senescence, in vivo monitoring, and safety-related clinical translational research still require in-depth investigation. This review summarizes the progress in pathological mechanisms and interventions, providing theoretical support for precision medicine targeting senescence, which is of great significance for addressing health challenges associated with aging.
Keywords: SASP; cardiovascular disease; cellular senescence; mechanisms; musculoskeletal disease; neurodegenerative disease; prevention–treatment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Mitochondrial dysfunction in the regulation of aging and aging-related diseases.Cell Commun Signal. 2025 Jun 19;23(1):290. doi: 10.1186/s12964-025-02308-7. Cell Commun Signal. 2025. PMID: 40537801 Free PMC article. Review.
-
Mechanisms of aging-related secretory phenotype regulation in osteoporosis: Network regulation, trade-offs and homeostasis.Pathol Res Pract. 2025 Aug;272:156115. doi: 10.1016/j.prp.2025.156115. Epub 2025 Jul 9. Pathol Res Pract. 2025. PMID: 40652750 Review.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Chk2 deletion rescues bone loss and cellular senescence induced by Bmi1 deficiency via regulation of Cyp1a1.J Orthop Translat. 2025 May 10;52:360-375. doi: 10.1016/j.jot.2025.04.014. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40698069 Free PMC article.
References
-
- SenNet Recommendations for Detecting Senescent Cells in Different Tissues|Nature Reviews Molecular Cell Biology. [(accessed on 23 May 2025)]. Available online: https://www.nature.com/articles/s41580-024-00738-8. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

