Novel Benzenesulfonamide Derivatives of 5'-Aminospirotriazolotriazine Exhibit Anti-Inflammatory Activity by Suppressing Pro-Inflammatory Mediators: In Vitro and In Vivo Evaluation Using a Rat Model of Carrageenan-Induced Paw Edema
- PMID: 40722802
- PMCID: PMC12292862
- DOI: 10.3390/biomedicines13071732
Novel Benzenesulfonamide Derivatives of 5'-Aminospirotriazolotriazine Exhibit Anti-Inflammatory Activity by Suppressing Pro-Inflammatory Mediators: In Vitro and In Vivo Evaluation Using a Rat Model of Carrageenan-Induced Paw Edema
Abstract
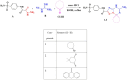
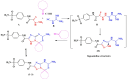
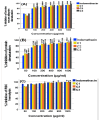
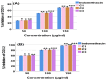
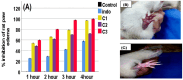
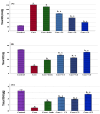
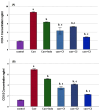
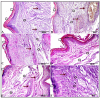
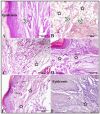
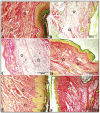
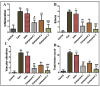
Background/Objectives: Inflammation is a crucial and complex mechanism that protects the body against infections. In our study, we propose to provide scientific evidence for the anti-inflammatory properties of 1,3,5-triazine derivatives. Methods: Initially, we ensured the safety of the three synthesized derivatives by administering graded doses of up to 2000 mg/kg intraperitoneally in Wistar rats. Thus, the three derivatives were considered generally safe. We also evaluated their ability to reduce carrageenan-induced rat paw edema. Results: Compounds 1, 2, and 3 demonstrated stronger anti-inflammatory activity than indomethacin (10 mg/kg), achieving maximum inhibition at the fourth hour with percentages of 96.31%, 72.08%, and 99.69%, respectively, at a dose of 200 mg/kg, compared to 57.66% for the standard drug. To explore the mechanism, levels of pro-inflammatory cytokines (TNF-α, IL-1α, IL-1β, IL-6, CRP) and oxidative stress markers were measured in paw tissue. All three compounds significantly reduced these markers more effectively than indomethacin and enhanced antioxidant levels (SOD and GSH) beyond those achieved by the standard treatment. Additionally, the compounds reduced COX-1 and COX-2 levels to values comparable to those in the normal (non-inflamed) control group. Conclusions: Compounds 1, 2, and 3 at doses of 200 mg/kg significantly (p < 0.05) inhibited the heat-induced hemolysis of red blood cell (RBC) membranes by 94.6%, 93.9%, and 95.2%, respectively, compared to 94.5% produced by indomethacin. Consequently, we concluded that 1,3,5-triazine derivatives are a safe antioxidant agent with significant anti-inflammatory activity.
Keywords: 1,3,5-triazine derivatives; MTT assay; anti-inflammatory; carrageenan; cox isoenzymes; indomethacin.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
New natural pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) and iNOS inhibitors identified from Penicillium polonicum through in vitro and in vivo studies.Int Immunopharmacol. 2023 Apr;117:109940. doi: 10.1016/j.intimp.2023.109940. Epub 2023 Mar 10. Int Immunopharmacol. 2023. PMID: 37012863
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Corticosteroids for the treatment of Duchenne muscular dystrophy.Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4. Cochrane Database Syst Rev. 2016. PMID: 27149418 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Moore R.A., Derry S., Phillips C.J., McQuay H.J. Nonsteroidal anti-inflammatory drugs (NSAIDs), cyxlooxygenase-2 selective inhibitors (coxibs) and gastrointestinal harm: Review of clinical trials and clinical practice. BMC Musculoskelet. Disord. 2006;7:1–13. doi: 10.1186/1471-2474-7-79. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

