Pulmonary Hemorrhage in Premature Infants: Pathophysiology, Risk Factors and Clinical Management
- PMID: 40722815
- PMCID: PMC12292498
- DOI: 10.3390/biomedicines13071744
Pulmonary Hemorrhage in Premature Infants: Pathophysiology, Risk Factors and Clinical Management
Abstract
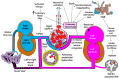
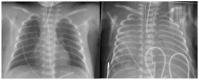
Pulmonary hemorrhage (PH) is a life-threatening complication predominantly affecting preterm infants, particularly those with very low birth weight (VLBW) and fetal growth restriction (FGR). Typically occurring within the first 72 h of life, PH is characterized by acute respiratory deterioration and significant morbidity and mortality. This review synthesizes current evidence on the multifactorial pathogenesis of PH, highlighting the roles of immature pulmonary vasculature, surfactant-induced hemodynamic shifts, and left ventricular diastolic dysfunction. Key risk factors include respiratory distress syndrome (RDS), hemodynamically significant patent ductus arteriosus (hsPDA), sepsis, coagulopathies, and genetic predispositions. Diagnostic approaches incorporate clinical signs, chest imaging, lung ultrasound, and echocardiography. Management strategies are multifaceted and include ventilatory support-particularly high-frequency oscillatory ventilation (HFOV)-surfactant re-administration, blood product transfusion, and targeted hemostatic agents. Emerging therapies such as recombinant activated factor VII and antifibrinolytics show promise but require further investigation. Preventive measures like antenatal corticosteroids and early indomethacin prophylaxis may reduce incidence, particularly in high-risk populations. Despite advancements in neonatal care, PH remains a major contributor to neonatal mortality and long-term neurodevelopmental impairment. Future research should focus on individualized risk stratification, early diagnostic tools, and optimized treatment protocols to improve outcomes. Multidisciplinary collaboration and innovation are essential to advancing care for this vulnerable population.
Keywords: coagulopathy; echocardiography; fetal growth restriction (FGR); lung ultrasound; patent ductus arteriosus (PDA); preterm infants; pulmonary hemorrhage; respiratory distress syndrome (RDS); surfactant therapy; very low birth weight (VLBW).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Zahr R.A., Ashfaq A., Marron-Corwin M. Neonatal Pulmonary Hemorrhage. NeoReviews. 2012;13:e302–e306. doi: 10.1542/neo.13-5-e302. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

