Negative Air Ions Attenuate Nicotine-Induced Vascular Endothelial Dysfunction by Suppressing AP1-Mediated FN1 and SPP1
- PMID: 40722963
- PMCID: PMC12292000
- DOI: 10.3390/antiox14070859
Negative Air Ions Attenuate Nicotine-Induced Vascular Endothelial Dysfunction by Suppressing AP1-Mediated FN1 and SPP1
Abstract
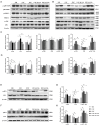
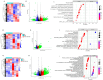
Nicotine-induced oxidative stress contributes significantly to vascular endothelial dysfunction. While negative air ions (NAIs) demonstrate potential blood-pressure-regulating and antioxidant properties, their mechanistic role remains unclear. This study examined the effects of NAIs against nicotine-induced oxidative damage and vascular endothelial injury in spontaneously hypertensive rats (SHRs). Western blotting was used to detect the expression levels of the α7nAChR/MAPK/AP1 pathway. Transcriptomic sequencing was performed to identify the differentially expressed genes after treatment with nicotine or NAIs. Furthermore, reactive oxygen species (ROS), endothelin-1 (ET-1), and [Ca2+]i levels were detected in human aortic endothelial cells (HAECs) treated with nicotine, and the relationship between transcription factor activator protein 1 (AP1) and the target genes was further elucidated through ChIP-qPCR. Nicotine exposure in SHRs elevated blood pressure and induced oxidative damage through α7nAChR/MAPK/AP1 pathway activation, causing endothelial structural disruption. These effects manifested as decreased NO/eNOS and increased ET-1/ETab expression, while these changes were reversed by NAIs. In HAECs, nicotine impaired proliferation while increasing oxidative stress and [Ca2+]i levels. This endothelial damage was markedly attenuated by either NAIs or fibronectin 1 (Fn1)/secreted phosphoprotein 1 (Spp1) knockdown. Mechanistically, we identified AP1 as the transcriptional regulator of FN1 and SPP1. NAIs attenuate nicotine-induced endothelial dysfunction in hypertension by inhibiting AP1-mediated FN1 and SPP1 activation, providing novel insights for smoking-associated cardiovascular risk.
Keywords: activator protein-1; hypertension; negative air ions; nicotine; vascular endothelial dysfunction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

