Inhaled and Systemic Steroids for Bronchopulmonary Dysplasia: Targeting Inflammation and Oxidative Stress
- PMID: 40722975
- PMCID: PMC12291945
- DOI: 10.3390/antiox14070869
Inhaled and Systemic Steroids for Bronchopulmonary Dysplasia: Targeting Inflammation and Oxidative Stress
Abstract
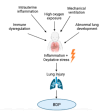
Bronchopulmonary dysplasia (BPD) remains a significant complication of preterm birth, characterized by impaired alveolar and vascular development, chronic lung inflammation, and long-term respiratory morbidity. Corticosteroids, both systemic and inhaled, have been widely investigated as potential therapeutic agents due to their anti-inflammatory properties and their emerging role in modulating oxidative stress. This narrative review explores the current evidence regarding the use of inhaled and systemic corticosteroids in the prevention and management of BPD, analyzing their efficacy, safety profiles, and long-term outcomes. While systemic corticosteroids, particularly dexamethasone, have demonstrated benefits in reducing ventilator dependence and lung inflammation, concerns regarding adverse neurodevelopmental effects have limited their routine use. Inhaled steroids have been proposed as a safer alternative, but their role in altering the disease trajectory remains controversial. A better understanding of the optimal timing, dosage, and patient selection is essential to maximize benefits while minimizing risks. Future research should focus on optimizing dosing strategies and identifying subgroups of preterm infants who may derive the greatest benefit from corticosteroid therapy.
Keywords: bronchopulmonary dysplasia; inflammation; inhaled steroids; lung injury; newborn; oxidative stress; systemic steroids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Inhaled versus systemic corticosteroids for the treatment of bronchopulmonary dysplasia in ventilated very low birth weight preterm infants.Cochrane Database Syst Rev. 2017 Oct 16;10(10):CD002057. doi: 10.1002/14651858.CD002057.pub4. Cochrane Database Syst Rev. 2017. PMID: 29035425 Free PMC article.
-
Inhaled versus systemic corticosteroids for preventing bronchopulmonary dysplasia in ventilated very low birth weight preterm neonates.Cochrane Database Syst Rev. 2017 Oct 17;10(10):CD002058. doi: 10.1002/14651858.CD002058.pub3. Cochrane Database Syst Rev. 2017. PMID: 29041034 Free PMC article.
-
Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD002057. doi: 10.1002/14651858.CD002057.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 May 16;(5):CD002057. doi: 10.1002/14651858.CD002057.pub3. PMID: 17943765 Updated.
-
Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants.Cochrane Database Syst Rev. 2003;(2):CD002057. doi: 10.1002/14651858.CD002057. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2007 Oct 17;(4):CD002057. doi: 10.1002/14651858.CD002057.pub2. PMID: 12804423 Updated.
-
Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants.Cochrane Database Syst Rev. 2012 May 16;(5):CD002057. doi: 10.1002/14651858.CD002057.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Oct 16;10:CD002057. doi: 10.1002/14651858.CD002057.pub4. PMID: 22592682 Updated.
References
-
- Jensen E.A., Dysart K., Gantz M.G., McDonald S., Bamat N.A., Keszler M., Kirpalani H., Laughon M.M., Poindexter B.B., Duncan A.F., et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019;200:751–759. doi: 10.1164/rccm.201812-2348OC. - DOI - PMC - PubMed
-
- Strashun S., Seliga-Siwecka J., Chioma R., Zielińska K., Włodarczyk K., Villamor E., Philip R.K., Assaf N.A., Pierro M. Steroid use for established bronchopulmonary dysplasia: Study protocol for a systematic review and meta-analysis. BMJ Open. 2022;12:e059553. doi: 10.1136/bmjopen-2021-059553. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

