Ethanolic Extract of Glycine Semen Preparata Prevents Oxidative Stress-Induced Muscle Damage in C2C12 Cells and Alleviates Dexamethasone-Induced Muscle Atrophy and Weakness in Experimental Mice
- PMID: 40722986
- PMCID: PMC12292089
- DOI: 10.3390/antiox14070882
Ethanolic Extract of Glycine Semen Preparata Prevents Oxidative Stress-Induced Muscle Damage in C2C12 Cells and Alleviates Dexamethasone-Induced Muscle Atrophy and Weakness in Experimental Mice
Abstract
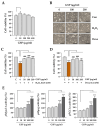
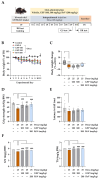
Skeletal muscle atrophy is a debilitating condition characterized by the loss of muscle mass and function. It is commonly associated with aging, chronic diseases, disuse, and prolonged glucocorticoid therapy. Oxidative stress and catabolic signaling pathways play significant roles in the progression of muscle degradation. Despite its clinical relevance, few effective therapeutic options are currently available. In this study, we investigated the protective effects of an ethanolic extract of Glycine Semen Preparata (GSP), i.e., fermented black soybeans, using in vitro and in vivo models of dexamethasone (Dexa)-induced muscle atrophy. In C2C12 myoblasts and myotubes, GSP significantly attenuated both oxidative stress-induced and Dexa-induced damages by reducing reactive oxygen species levels and by suppressing the expression of the muscle-specific E3 ubiquitin ligases MuRF1 and Atrogin-1. Moreover, GSP upregulated key genes involved in muscle regeneration (Myod1 and Myog) and mitochondrial biogenesis (PGC1α), indicating its dual role in muscle protection and regeneration. Oral administration of GSP to mice with Dexa-induced muscle atrophy resulted in improved muscle fiber integrity, increased proportion of large cross-sectional area fibers, and partial recovery of motor function. Isoflavone aglycones, such as daidzein and genistein, were identified as active compounds that contribute to the beneficial effects of GSP through antioxidant activity and gene promoter enhancement. Thus, GSP is a promising nutraceutical that prevents or mitigates muscle atrophy by targeting oxidative stress and promoting myogenesis and mitochondrial function. Further studies are warranted to standardize the bioactive components and explore their clinical applications.
Keywords: Glycine Semen Preparata; fermented black soybean; glucocorticoid; muscle atrophy; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Umbelliferone attenuates diabetic sarcopenia by modulating mitochondrial quality and the ubiquitin-proteasome system.Phytomedicine. 2025 Aug;144:156930. doi: 10.1016/j.phymed.2025.156930. Epub 2025 May 31. Phytomedicine. 2025. PMID: 40483791
-
Bu-zhong-yi-qi decoction regulates JNK/c-JUN signaling pathway to improve skeletal muscle atrophy caused by cancer cachexia.J Ethnopharmacol. 2025 Jul 24;351:120078. doi: 10.1016/j.jep.2025.120078. Epub 2025 Jun 1. J Ethnopharmacol. 2025. PMID: 40460921
-
Yuja peel hot water extract protects against dexamethasone-induced skeletal muscle atrophy through the PI3K-Akt-mTOR/FoxO3α signaling pathway.Nutr Res Pract. 2025 Aug;19(4):511-524. doi: 10.4162/nrp.2025.19.4.511. Epub 2025 Apr 23. Nutr Res Pract. 2025. PMID: 40809884 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Immunomodulatory potential of dietary soybean-derived saponins.J Anim Sci. 2024 Jan 3;102:skae349. doi: 10.1093/jas/skae349. J Anim Sci. 2024. PMID: 39529449 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

